Journal of Toxins
Download PDF
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology and Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 A P C Road, Kolkata 700 009, India, Tel: 91-33-23508386/ (M) +919433139031/+919674544016; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Ghosh S, Gomes A. Russell´s viper (Daboia Russelli Russelli) Venom Toxicity Neutralizing Efficacy of Curcumin- Gold Nanoparticle (C-GNP) in Experimental Animal Model. J Toxins. 2016;3(2): 6
Copyright © 2016 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 2
Submission: 08 September, 2016 | Accepted: 21 October, 2016 | Published: 30 October, 2016
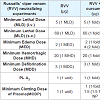
RVV neutralization
C. Neutralization of minimum hemorrhagic dose: In vivo RVV-induced Minimum Hemorrhagic Dose (MHD) was found to be 20 µg/20 gm/i.d/24 hrs in male albino mice. Curcumin (50 µg) failed to offer any protection against RVV-induced hemorrhage, whereas C-GNP gave 1 fold protection against RVV-induced hemorrhagic activity in Male Albino Mice (Table 1 and Figure 2).
Russell´s viper (Daboia Russelli Russelli) Venom Toxicity Neutralizing Efficacy of Curcumin- Gold Nanoparticle (C-GNP) in Experimental Animal Model
Antony Gomes* and Sourav Ghosh
- Laboratory of Toxinology and Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, India
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology and Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 A P C Road, Kolkata 700 009, India, Tel: 91-33-23508386/ (M) +919433139031/+919674544016; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Ghosh S, Gomes A. Russell´s viper (Daboia Russelli Russelli) Venom Toxicity Neutralizing Efficacy of Curcumin- Gold Nanoparticle (C-GNP) in Experimental Animal Model. J Toxins. 2016;3(2): 6
Copyright © 2016 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 2
Submission: 08 September, 2016 | Accepted: 21 October, 2016 | Published: 30 October, 2016
Abstract
Herbs/herbal compounds possess anti-snake venom activity. Anti-snake venom activity of Curcuma longa in animal model has been reported earlier. In the present study, Curcumin (C), an active ompound present in Curcuma longa was Conjugated with Gold Nanoparticle (C-GNP) and its neutralizing efficacy against viper (Daboia russelli russelli) venom induced toxicity was evaluated in animal models. C-GNP was synthesized by adsorption method using chemically synthesized gold nanoparticle. Physicochemical characterization of C-GNP was done by DLS (size + zeta potential), UV-visible spectra, XRD and FESEM. Snake venom neutralizing activity of curcumin/C-GNP was done using in vivo and in vitro models. Animals were divided into gr. 1: Sham control, gr. 2: RVV control, gr. 3: Curcumin treated and gr. 4: C-GNP treated. Animal ethical clearance was availed before experiments (IAEC/IV/Proposal/AG- 001/2015; Dt: 10.04.2015). One way ANOVA was done (n=4) and Pin vitro) was observed after C-GNP treatment. The present study confirmed the formation of C-GNP and its efficacy against RVV induced toxicity. It may act as supportive treatment of snake bite victims. Further detailed studies on the antivenom activity and molecular mechanism of C-GNP are warranted.Keywords
Snake venom; Russells´ viper venom; Snake venom neutralization; Curcumin; Gold nanoparticleIntroduction
Russell´s viper envenomation is associated with local swelling, incoagulable blood, thrombocytopenia, spontaneous systemic bleeding, hypotension, increased capillary permeability, oliguria, proteinuria and urinary blood loss [1]. The only available treatment for RVV is anti snake RVV (ASVS), which was first developed in 1894. In viper envenomation, rapid initial absorption followed by slow absorption of venom. The delayed absorption has been linked with recurrence of envenomation when ASVS levels in blood decreases [2]. ASVS cannot neutralize viper venom induced local effects such as swelling, spontaneous systemic bleeding, increased capillary permeability, incoagulable blood, oliguria, thrombocytopenia, hypotension, proteinuria and urinary blood loss [3]. ASVS treatment carries a high risk of serum sickness and a lesser risk of anaphylaxis [4]. There is a need for alternative therapeutics that can effectively neutralize RVV induced toxicities. From the present laboratory, many herbal antidotes have been identified against viper and cobra venom tested in animal models [4-13]. Recently, Gomes et al. reported that efficacy of herbal compound against RVV was increased by gold nanoparticle conjugation [14]. Use of nanotechnology increases the efficacy and bioavailability of drugs [15]. Karain et al. has shown that C60 fullerene nanoparticles decreased the lethality of Pacific Rattle snake venom in Acheta domesticus model [16]. In the present study, curcumin (an active compound present in the rhizomes of Curcuma longa) was conjugated with gold nanoparticle and its Russell´s viper venom (RVV)-induced toxicity neutralizing efficacy was studied in animal model.Materials and Methods
Venom: Lyophilized RVV was purchased from Calcutta Snake Park, Kolkata, India and preserved in desiccators at 4 °C in an amber colored glass vial until further use. It was dissolved in Milli-Q water, kept at 2-8 °C for overnight and centrifuged at 3000 rpm for 10 min. The supernatant was used as venom and kept at 2-8 °C until further use. The venom concentration was expressed in terms of dry weight (mg/ml, w/v).Herbal compound: Curcumin was obtained commercially from Sigma-Aldrich (USA, Catalogue No: C7727). It (10 mg) was dissolved in 1 ml absolute alcohol and diluted 10 times by slowly adding distilled water. The final concentration of curcumin solution was 1 mg/ml and expressed in terms of dry weight (mg/ml, w/v).
Animals: Swiss male albino mice (20±1 gm) aged 8 weeks were obtained from authorized animal suppliers of Calcutta University. The animals were kept in polypropylene cages, acclimatized and maintained in a controlled environment (temperature: 25±2 °C, humidity: 60±5% and 12 hr light/dark cycle). Food (pellet diet, Bengal gram and fresh green vegetables) and water was provided in ad libitum. All experimental protocols described in this study were approved by the animal ethics committee, Dept of Physiology, University of Calcutta and were in accordance with the guideline of the committee for the purpose of control and supervision of experiments on animal CPCSEA), Government of India, (Animal ethical clearance no: IAEC/IV/Proposal/AG-01/2015; Dated 10.04.2015). Swiss male albino mice (20±1 gm) were divided into: Gr.1- sham control, Gr.2- RVV control, Gr.3-curcumin treated and Gr.4- C-GNP treated.
Gold Nanoparticle Conjugation with Curcumin (C-GNP): Gold salt was purchased from Sigma-Aldrich (USA, Catalogue No: 484385). Gold Nanoparticles (GNP) were synthesized by citrate reduction method [17]. Briefly, an aqueous solution of HAuCl4 (250 µM, 25 mL) was brought to almost boiling condition up to 100 °C stirring continuously on a magnetic stirrer. Freshly prepared trisodium isocitrate solution (600 µM final concentration) was added quickly to it which brought change in the solution color from pale yellow to bluish and finally to deep red. After obtaining a stable deep red color, the solution was allowed to be cooled in room temperature with continuous stirring. Gold nanoparticle prepared was then conjugated with curcumin to synthesize curcumin-conjugated GNP (C-GNP) by adsorption method. 1 mL of Curcumin (1 mg/mL) was added slowly to 2 mL of freshly prepared GNP on a vortex mixture, kept at 4 °C for further use, and termed as C-GNP. To check the stability of the nanoparticles, it was kept at different temperatures for 3 months: at room temperature (28±3 °C), at incubator (37±1 °C) and at refrigerator (8±1 °C).
Characterization of C-GNP: UV-visible spectra of curcumin and C-GNP were measured at the range between 200 nm to 700 nm using spectrophotometer (Shimadzu UV-1800) to determine maximum absorbance of respective solutions. The hydrodynamic diameter and zeta potential of C-GNP was measured by using photon correlation spectroscope or Dynamic Light Scattering (DLS) machine (Beckmann Coulter Delsa Nano C TM) to determine its size and stability. XRD measurements of C-GNP was done by XRD analyser (Phillips PW 1830) operating at a voltage of 40 KV and current of 20 mA with Cu-K radiation. FESEM of C-GNP was done using scanning electron microscope (Zeiss EVO 18).
A. Neutralization of minimum lethal dose: The Minimum Lethal Dose (MLD) of RVV was assessed by injection of different amounts of RVV dissolved in 0.2 ml of 0.9% saline water into the tail vein of Swiss male albino mice (20±1 gm) after Theakston and Reid [18]. Various amounts of RVV (1-3 MLD) were mixed with fixed amount of curcumin/C-GNP incubated at 37 °C for 15 min and injected into the tail vein of Swiss male albino mice (n=4). The MLD neutralization capacity of curcumin/C-GNP was calculated after 24hrs of observation.
B. Neutralization of minimum edema dose: The Minimum Edema Dose (MED) of RVV was defined as the least amount of RVV when injected (intra-plantar) into Swiss male albino mice (20±1 gm), produced edema in the left paw (100% rise in paw volume). The right paw received saline 0.9% and was treated as control. To assess the antiedema activity, various doses of RVV (1-5 MED in 0.05 ml) incubated at 37 °C X 15 min with curcumin/C-GNP were injected (intra-plantar route) in Swiss male albino mice (n=4). The paw edema (volume) was recorded by the use of a digital caliper (Mitutua, Japan) at given time intervals (0,2,4 and 24 hrs), photographed by Canon digital camera (model no. SX 30 IS) and neutralization fold was calculated.
C. Neutralization of minimum hemorrhagic dose: The Minimum Hemorrhagic Dose (MHD) of RVV was defined as the least amount of RVV when injected intradermal (i.d.) into Swiss male albino mice (20±1 gm), produced cutaneous hemorrhage of 10 mm diameter after 24 hrs of observation [18]. To assess the antihemorrhagic activity, various doses of RVV (1-3 MHD in 0.05 ml) incubated at 37 °C for 15 min with curcumin/C-GNP were injected (i.d.) in male Swiss albino mice (n=4). After 24 hrs, the animals were sacrificed, skin was removed and hemorrhagic spot was measured by the use of a digital calliper (Mitutua, Japan). Neutralization fold was calculated, photographs were taken by Canon digital camera (model no. SX 30 IS).
D. Neutralization of minimum defibrinating dose: The Minimum Defibrinating Dose (MDD) was defined as the minimum amount of RVV which, when injected intravenously (i.v.) into male albino mice (20±1 gm, n=4) produces incoagulable blood 2 hour later [18]. Inhibition of this activity was estimated by injecting different amount of RVV (i.v.) and curcumin (50 mg/kg/i.p.) or C-GNP (200 µl/20 gm mice/i.p.). The nature of the blood (clotted/non-clotted) was observed after 2 hour.
E. Neutralization of RVV phospholipase A2 activity: RVV phospholipase A2 (PLA2) activity was estimated by egg yolk coagulation method of Habermann and Neumann [19]. One unit of enzyme activity was defined as the amount of RVV which increased the coagulation time of egg yolk by 1 min. For neutralization of the RVV PLA2 activity, RVV (1-8 units) was incubated with curcumin/ C-GNP at 37 °C for 15 min and PLA2 activity was assayed. Fold of RVV PLA2 neutralization was calculated in terms of PLA2 units.
F. Neutralization of RVV-induced minimum clotting dose of plasma: The Minimum Clotting Dose of Plasma (MCDP) induced by RVV was determined by Theakston and Reid [18]. Goat plasma was obtained from Government slaughter house in 3.8% sodium citrate in a ratio 1:9 (v/v). Plasma (0.2 ml) was incubated in a water bath at 37 °C. To each tube, 0.1 ml of RVV in different concentrations (0.1 ml of saline added in control) was added. Finally 0.1 ml of 25 mM CaCl2 was added and clotting time was recorded with the help of a stop watch. Neutralization of RVV coagulant activity was done by mixing RVV (1-2 MCDP) with the fixed amount of curcumin/C-GNP at 37 °C X 15 min and the clotting time was recorded.
G. Neutralization of RVV-induced serum markers: Animals were divided into Gr.1- sham control, Gr.2- RVV control, Gr.3- curcumin treated and Gr.4- C-GNP treated. Twelve µg (1/5th of MLD through s.c. route) of RVV was injected in Gr. 2,3 and 4 animals. Gr. 3&4 animals were treated with curcumin (50 mg/kg/s.c.) & C-GNP (100 µl/20 gm/s.c.), respectively. After 18 hours, blood was collected from orbital plexus of animals, serum was prepared and creatinine, urea, Lactate Dehydrogenase (LDH), Serum Glutamic Oxaloacetic Transaminase (SGOT) and serum Acid Phosphatase (ACP) were estimated by biochemical kit method (Spinreact, Spain) according to manufacturer´s instruction.
Statistical analysis: Statistical significance was evaluated by one way Analysis of Variance (ANOVA). Values were expressed as mean ± standard error of mean (n=4) and P
Statistics
All results were expressed as mean±standard error of mean (n=6). The level of significance of difference between means was determined by Student´s t test and P
Results
Formation and characterization of C-GNP: C-GNP´s formed as orange color. It was visibly stable at room temperature (28±3 °C) for 15±3 days, incubator (37±1 °C) for 20±3 days and refrigerator (8±1 °C) for 60±5 days. Zeta potential of C-GNP was found to be -26.11 mV. λmax of curcumin and C-GNP was found to be 420 nm and 538 nm, respectively. When excited at 420 nm, curcumin showed fluorescent activity. C-GNP showed a quenching in fluorescence activity after excited at 420 nm. Hydrodynamic diameter of C-GNP was found to be 230-260 nm with polydispersity index of 0.103. X-ray diffraction analysis indicated that C-GNP was composed of crystalline gold. The Field Emission Scanning Electron Microscopy (FESEM) data of C-GNP showed that the particle size was 18-24 nm with nearly spherical shape Figure 1.RVV neutralization
A. Neutralization of minimum lethal dose: RVV Minimum Lethal Dose (MLD) was found to be 5 µg/20 gm/i.v and 60 µg/20 gm/ s.c. in male Swiss albino mice. Curcumin and C-GNP did not have any protection against RVV-induced lethality in male albino mice through intravenous and subcutaneous route Table 1.
B. Neutralization of minimum edema dose: In vivo RVV Minimum Edema Dose (MED) was found to be 2 µg/20 gm/intraplanter. Curcumin gave 1 fold protection against RVV-induced edema, whereas, C-GNP caused 2 fold protection. The edema induced by RVV over a time period of 24 h was significantly neutralized by C-GNP as compared with curcumin. Curcumin gave 10.65% protection in RVV-induced paw edema after 24 h, whereas C-GNP gave 23.82% protection after 24 h Table 1.
C. Neutralization of minimum hemorrhagic dose: In vivo RVV-induced Minimum Hemorrhagic Dose (MHD) was found to be 20 µg/20 gm/i.d/24 hrs in male albino mice. Curcumin (50 µg) failed to offer any protection against RVV-induced hemorrhage, whereas C-GNP gave 1 fold protection against RVV-induced hemorrhagic activity in Male Albino Mice (Table 1 and Figure 2).
D. Neutralization of minimum defibrinating dose: In vivo Minimum Defibrinating Dose (MDD) of RVV was found to be 2 µg. Treatment with curcumin offered no protection in RVV-induced defibrination activity, whereas treatment with C-GNP offered 1 fold protection in RVV-induced defibrinating activity Table 1.
E. Neutralization of RVV-induced phospholipase A2 activity: In vitro RVV-induced 1 unit phospholipase A2 activity was found to be 1 µg. Curcumin offered no protection in RVV-induced PLA2 activity. C-GNP was found to neutralize 1 unit of PLA2 activity Table 1.
F. Minimum clotting dose of plasma: In vitro RVV-induced Minimum Clotting Dose of Plasma (MCDP) was found to be 1 µg. Curcumin offered 1 fold protection in RVV-induced MCDP. There was 1.5 fold protection in MCDP after treatment with C-GNP Table 1.
G. Neutralization of RVV-induced serum markers: There was significant increase in serum urea (53.5%, P<0.01), serum creatinine (41.8%, P<0.05), serum lactate dehydrogenase (78.0%, P<0.01), serum glutamic oxaloacetic transaminase (198.5%, P<0.001) and serum acid phosphatase (84.2%, P<0.01) in group 2 RVV control animals as compared with group 1 sham control animals. Treatment with curcumin in group 3 animals caused significant decrease in serum urea (14.3%, P<0.05), serum creatinine (16.8%, P<0.05), serum lactate dehydrogenase (20.6%, P<0.05), serum glutamic oxaloacetic transaminase (37.6%, P<0.05) and serum acid phosphatase (23.4%, P<0.05) as compared with group 2 RVV control animals. Treatment with C-GNP in group 4 animals caused significant decrease in serum urea (28.9%, P<0.05), serum creatinine (25.3%, P<0.05), serum lactate dehydrogenase (32.4%, P<0.01), serum glutamic oxaloacetic transaminase (50.0%, P<0.01) and serum acid phosphatase (34.1%, P<0.01) as compared with group 2 RVV control animals Figure 3.
Figure 3: Effect of C-GNP in RVV induced changes in serum markers in animal model. Significant change was observed in serum urea, creatinine, Lactate Dehydrogenase (LDH), Serum Glutamic Oxaloacetic Transaminase (SGOT) and serum Acid Phosphatase (ACP) after treatment with C-GNP in RVV-induced animals. *P<0.05; **P<0.01. Values were expressed as mean ± SEM; Gr.1- sham control, Gr.2- RVV control, Gr. 3- curcumin treated, Gr.4- C-GNP treated.
Discussion
ASVS, developed by Dr. Albert Calmett in 1894 is the only treatment for snake bite till date, and there is no alternative available. However, there are many limitations (storage problem, availability in rural areas, dose difficulties, cost factors) and side effects (pyrogenicity, serum sickness, anaphylactic reactions etc.) of using ASVS [20-22]. Many herbs and herbal compounds have been identified to be active against snake venom [12]. Sometimes pure herbal compounds are less effective than the whole herbal extract. So, the question arises: Whether we can increase the efficacy and decrease the toxicity of the anti-snake venom herbal compounds using nanotechnology? Gold anoparticle is a potential candidate in the field of nanomedicine and nanotherapeutics. In Ayurveda (Indian traditional medicinal system), processed gold and other metals were used as preventive and curative agents against many diseases. Kulkarni has established that ayurvedic processing of metals converted them into nanoparticles [23]. In traditional times, mixture of metallic formulation and herbs/herbal products were very common practice in therapeutics. Gomes et al. Ghosh and Saha have established the increase in efficacy of herbs/ herbal constituents after conjugation of gold nanoparticle [14,24-26]. The present study was aimed to introduce a new approach of viper envenomation management (supportive therapy to ASVS) use of C-GGNP´s in animal models.Several methods that are available for nanoparticle conjugation are adsorption or sodium borohydrate method, which increases the efficacy of the herbs/herbal products. Curcumin was first made soluble in alcohol, and then was adsorbed in gold nanoparticle. The physicochemical characterizations of C-GNP were done to speculate the stability, size, shape and conjugation of the particle. Stability study of C-GNP was done by estimating zeta potential of the particles using dynamic light scattering technique. It is the potential difference existing between the particle surfaces and dispersing liquid. Gold nanoparticles bear negative charges; higher negative charge will cause more repulsion force between the particles along with more stability. The result indicated the formation of stable particles in room temperature (28±3 °C), incubator (37±1 °C) and refrigerator (8±1 °C). UV-vis spectrum is sensitive to particle size, shape, local refractive index and its interaction with medium [27]. Curcumin emits fluorescence when excited at its maximum absorbance. After conjugation with nanoparticles, they show fluorescence quenching activity. Joshi et al. showed that, after conjugation there was quenching of fluorescent activity of nanoparticles [28]. Dynamic Light Scattering (DLS) is used to determine the surface distribution profile of small particles in suspension, and it measures its hydrodynamic diameter, measuring the fluctuation of scattered light intensity of small sized particles [29]. Number of peaks of particle size determines its monodispersive or polydispersive nature. Polydispersity index is the parameter by which the particle-size differences can be estimated. In the present study, low polydispersity index confirms the formation of monodispersive C-GNP. Structural characterization of C-GNP was done by X-ray diffraction analysis and the result indicated the presence of crystalline gold in C-GNP. The Field Emission Scanning Electron Microscopy (FESEM) data of C-GNP showed the spherical shaped particle with size 18-24 nm. There was a difference between DLS diameter and FESEM diameter, because DLS diameter measures the water and other solute molecules attached with the particle, whereas the FESEM diameter measures the actual particle´s diameter.
Russell´s viper, mostly found in Indian subcontinent, South-East Asia and China causes a number of deaths throughout the year. The possible mechanism of action for lethality of RVV includes hemolysis, pre-synaptic neurotoxicity, rhabdomyolysis, vasodialation and shock [30]. About 70% of protein content in RVV is phospholipase A2 with at least 7 isoenzyme forms [30]. High phospholipase activity is associated with high lethality [31]. Zinc-dependent metalloproteinases present in RVV are largely responsible for the hemorrhagic damage [32]. The hemorrhagic toxins directly damage microvessels and cause hemostasis, which provoke profuse bleeding [33,34]. RVV induced hemorrhage is also associated with pathophysiological manifestations including local necrosis, edema and blisters [32].
Earlier studies indicated that curcumin can inhibit phospholipases, metalloproteinases and protein kinase C [35-38] that may act effectively against snake bite. In the present study, the efficacy of curcumin was increased by tagging with gold nanoparticle. C-GNP did not offer any protection against RVV-induced lethality in animal model, but it neutralized RVV induced edema, defibrination and hemorrhage. Study of venom induced in vitro phospholipase A2 activity and minimum clotting dose of plasma mimics the clinical manifestations of snake bite. C-GNP offers significant protection in RVV induced phospholipase A2 activity and clotting dose of plasma. RV envenomation causes nephrotoxicity, hepatotoxicity and myotoxicity in animal model [26]. Raised levels of urea, creatinine (for nephrotoxicity), AST, ACP (for hepatotoxicity) and LDH (for myotoxicity) were seen in RVV control animals. These markers were significantly reduced after treatment with C-GNP indicating that C-GNP inhibited RVV induced organ damages (liver, kidney, muscle) in animal models.
Previous studies indicated that curcumin has antioxidant and anti-inflammatory properties that help in exerting its protective role in pathophysiology [39]. Saha et al, Gomes et al. has shown that conjugation of gold nanoparticle with herbs/herbal compounds increased their potential against viper and cobra venom in animal model [14,25]. At this stage, it is difficult to point out the molecular mechanism of RVV neutralization by C-GNP, although the in vivo and in vitro studies showed some interesting data which confirmed the effective neutralization of RVV induced local toxic effects. It is likely that C-GNP acts at (1) neutralizing the RVV induced local damages at the vascular bed by inhibiting the pro-oxidant activity of RVV, (2) direct inhibition at the enzymatic level, (3) interference with the cellular markers (proinflammatory markers, antioxidants etc.) (Ghosh and Gomes, unpublished data). Although C-GNP could not neutralize RVV induced lethality, it may act as supportive treatment of snake bite victims. The present study confirmed the effective conjugation of gold nanoparticle with curcumin, which neutralized RVV-induced local toxicity in animal models, thus providing clue for supportive therapy along with ASVS treatment.
Conclusion
Snake venom neutralization using nanotechnology is a new domain of biomedical research which is showing promises for the development of supportive anti snake venom antidote from herbal resources.Acknowledgements
The authors are indebted to Indian Council of Medical Research (ICMR), New Delhi, India (Ref. No- 58/7/2002, dt. 20.07.2006) for partial funding and to Prof. N. Paria, Department of Botany, Calcutta University, Calcutta, India for identification of the plant.References
- Myint-Lwin, Warrell DA, Phillips RE, Tin-Nu-Swe, Tun-Pe, et al. (1985) Bites by Russell's viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet 2:1259-1264.
- de Gutierrez JM, Leon G, Lomonte B (2003) Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinet 42: 721-741.
- Santhosh MS, Hemshekhar M, Sunitha K, Thushara RM, Jnaneshwari S, et al. (2013) Snake venom induced local toxicities: plant secondary metabolites as an auxiliary therapy. Mini Rev Med Chem 13:106-123.
- Nelson BK (1989) Snake envenomation. Incidence, clinical presentation and management. Med Toxicol Adverse Drug Exp 4: 17-31.
- Alam MI, Auddy B, Gomes A (1994) Isolation, purification and partial characterization of viper venom inhibiting factor from the root extract of the Indian medicinal plant sarsaparilla (Hemidesmus indicus R. Br.). Toxicon 32:1551-1557.
- Alam MI, Auddy B, Gomes A (1996) Viper venom neutralizing by indian medicinal plant (Hemidesmus indicus and Pluchea indica) root extract. Phytother Res 10: 58-61.
- Alam MI, Gomes A (1998) Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantamul (Hemidesmus indicus R. BR) root extract. Toxicon 36: 207-215.
- Alam MI, Gomes A (1998) Adjuvant effects and antiserum action potentiation by a (herbal) compound 2-hydroxy-4-methoxy benzoic acid isolated from the root extract of the Indian medicinal plant 'sarsaparilla' (Hemidesmus indicus R. Br.). Toxicon 36: 1423-1431.
- Alam MI, Gomes A (2003) Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol 86: 75-80.
- Chatterjee I, Chakravarty AK, Gomes A (2006) Daboia russellii and Naja kaouthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R.Br. J Ethnopharmacol 106: 38-43.
- Gomes A, Saha A, Chatterjee I, Chakravarty AK (2007) Viper and cobra venom neutralization by beta-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae). Phytomedicine 14: 637-643.
- Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, et al. (2010) Herbs and herbal constituents active against snake bite. Indian J Exp Biol 48: 865-878.
- Sarkhel S, Chakravarty AK, Das R, Aparna Gomes, Gomes A (2011) Snake venom neutralising factor from the root extract of Emblica officinalis Linn. Orient Pharm Exp Med 11: 25-33.
- Gomes A, Sengupta J, Ghosh S, Gomes A (2016) Application of gold nanoparticle conjugation with 2-Hydroxy-4-Methoxy Benzoic Acid (HMBA) from Hemidesmus indicus root enhancing neutralization of snake (Viper) venom activity. J Nanosci Nanotechnol 16: 8322-8329.
- Gomes A, Ghosh S, Sengupta J, Datta P, Gomes A (2014) Herbonanoceuticals: A New Step Towards Herbal Therapeutics. Med Aromat Plants 3: 162.
- Karain BD, Lee MK, Hayes WK (2016) C60 fullerenes as a novel treatment for poisoning and envenomation: A proof-of-concept study for snakebite. J Nanosci Nanotechnol 16: 7764-7771.
- Karain BD, Lee MK, Hayes WK (2016) C60 fullerenes as a novel treatment for poisoning and envenomation: A proof-of-concept study for snakebite. J Nanosci Nanotechnol 16: 7764-7771.
- Theakston RD, Reid HA (1983) Development of simple standard assay procedures for the characterization of snake venom. Bull World Health Organ 61: 949-956.
- Habermann E, Neumann N (1954) Egg yolk coagulation method. Physiol Chem 297: 174.
- Offerman SR, Smith TS, Derlet RW (2001) Does the aggressive use of polyvalent antivenin for rattlesnake bites result in serious acute side effects? West J Med 175: 88-91.
- Lalloo DG, Theakston RD (2003) Snake antivenoms. J Toxicol Clin Toxicol 41: 277-290, 317-327.
- Williams DJ, Jensen SD, Nimorakiotakis B, Muller R, Winkel KD (2007) Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon 49: 780-792.
- Kulkarni SS (2013) Bhasma and nanomedicine. Int Res J Pharm 4: 10-16.
- Ghosh S, Sengupta J, Datta P, Gomes A (2014) Hematopoietic and antioxidant activities of gnp synthesized by aqueous extract of Fenugreek (Trigonella foenum-graecum) Seed. Adv Sci Eng Med 6: 546-552.
- Saha K, Ghosh S, Ghosh S, Dasgupta SC, Gomes A, et al. (2015) Neutralization of naja kaouthia venom induced toxicity and stress response by vitex negundo-gold nanoparticle (VN-GNP) in Experimental Animal Model. J Toxins 2: 1-8.
- Saha K, Gomes A (2016) Russell´s viper venom induced nephrotoxicity myotoxicity, hepatotoxicity: neutralization with gold nanoparticle conjugated 2-hydroxy-4-methoxy benzoic acid (GNP-HMBA) in experimental animal models. Indian J Exp Biol (In Press).
- Aswathy Aromal S, Philip D (2012) Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc 97: 1-5.
- Joshi P, Shewale V, Pandey R, Shanker V, Hussain S, et al. (2011) Site specific interaction between ZnO nanoparticles and tryptophan: a first principles quantum mechanical study. Phys Chem Chem Phys 13: 476-479.
- Kaszuba M, McKnight D, Connah MT, McNeil-Watson FK, Nobbmann U (2008) Measuring sub nanometre sizes using dynamic light scattering. J Nanopart Res 10: 823-829.
- Warrell DA (1989) Snake venoms in science and clinical medicine. 1. Russell´s viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg 83: 732-740.
- Alam MI, Auddy B, Jayanthi GP, Gowda TV (1988) Geographical variation in India in the composition and lethal potency of Russell´s viper (Vipera russelli) venom. Toxicon 26: 257-264.
- Gutiérrez JM, Rucavado A, Escalante T, Díaz C (2005) Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 45: 997-1011.
- Bjarnason JB, Fox JW (1994) Hemorrhagic metalloproteinases from snake venoms. Pharmacol Ther 62: 325-372.
- Markland FS (1998) Snake venoms and the hemostatic system. Toxicon 36: 1749-1800.
- Yamamoto H, Hanada K, Kawasaki K, Nishijima M (1997) Inhibitory effect on curcumin on mammalian phospholipase D activity. FEBS Lett 417: 196-198.
- Hong J, Bose M, Ju J, Ryu JH, Chen X, et al. (2004) Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase.Carcinogenesis 25: 1671-1679.
- Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, et al. (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 285: 127-133.
- Lin JK, Chen YC, Huang YT, Lin-Shiau SY (1997) Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J CellBiochem Suppl 28-29: 39-48.
- Aggarwal BB, Harikumar KB (2009) Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41: 40-59.