Journal of Toxins
Download PDF
Research Article
*Address for Correspondence: Subir Chandra Das Gupta, Department of Zoology, Maulana Azad College, 8, Rafi Ahmed Kidwai Road, Kolkata 700013, India, Tel: 91-33-2226-0995; Fax-91-33-2226-0995 E-mail: subirdgupta@gmail.com
Citation: Mukherjee S, Gupta SCD, Gomes A. Estrous Cycle Cessation Caused via Activating the Apoptotic Pathway by NNSS2 Isolated from Naja Naja Shedded Skin. J Toxins. 2016;3(1): 3
Copyright © 2016 Mukherjee S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 1
Submission: 29 January, 2016 | Accepted: 09 March, 2016 | Published: 14 March, 2016
Western immunoblot analysis
Estrous Cycle Cessation Caused via Activating the Apoptotic Pathway by NNSS2 Isolated from Naja naja Shedded Skin
Sanghamitra Mukherjee1, Subir Chandra Das Gupta1*, and Antony Gomes2
- 1Department of Zoology, Maulana Azad College, 8, Rafi Ahmed Kidwai Road, Kolkata 700013, India
- 2Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, Calcutta University, 92, APC Road, Kolkata 700009, India
*Address for Correspondence: Subir Chandra Das Gupta, Department of Zoology, Maulana Azad College, 8, Rafi Ahmed Kidwai Road, Kolkata 700013, India, Tel: 91-33-2226-0995; Fax-91-33-2226-0995 E-mail: subirdgupta@gmail.com
Citation: Mukherjee S, Gupta SCD, Gomes A. Estrous Cycle Cessation Caused via Activating the Apoptotic Pathway by NNSS2 Isolated from Naja Naja Shedded Skin. J Toxins. 2016;3(1): 3
Copyright © 2016 Mukherjee S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 1
Submission: 29 January, 2016 | Accepted: 09 March, 2016 | Published: 14 March, 2016
Abstract
The snake shed skin has long been used in the Chinese and Indian traditional, folk medicine for various medicinal purposes. Previously it was reported that N. naja shedded skin caused temporary cessation of the estrous cycle. Based on these reviews and reports this work was undertaken to purify, characterize bioactive compound from Naja naja shedded skin and to assess its bioactivity in animal model.The N. naja shed skin extract was subjected to thin layer chromatography, HPLC for purification to obtain active factor, and it was characterized (MALDI, CD spectral analysis, H1NMR & XRD). Purified fraction was injected subcutaneously in female Swiss albino mice. Serum progesterone, estradiol, IL-1β, TNF-α levels were assessed; histopathology of uterus and ovary was done. Uterine and ovarian apoptotic marker expressions were studied.
NNSS2 was purified having molecular weight of 882.6 Dalton. It caused cessation of estrous cycle for 5 days, decreased serum progesterone, P4-E2 ratio, increased IL-1β, caspase 3,9, Bax, decreased BCL2, HSP70, HSP90 expressions and cleaved PARP. Histological studies of ovary showed immature follicles; and uterus displayed constricted structure. NNSS2 influenced reproductive cycle of female albino mice by altering hormones, cytokine profiles, apoptotic markers, and by involvement of intrinsic apoptotic pathway. This study provided a novel outline of the probable bioactive compounds present in the N. naja shedded skin.
Keywords
Anestrous; Folk and traditional medicine; Follicular atresia; Phase arrestIntroduction
The estrous cycle of female mice involved four phases: diestrous, metestrous, proestrous and estrous, it lasted for 4-5 days. It is regulated very intricately by endocrine and cytokine circuitry. The short length and less complexity of the estrous cycle in mice made it ideal animal experimental model for study on reproductive cycle, including the effect of anti fertility agents from natural sources.The female anti fertility drugs generally prescribed by doctors are chemically steroids, available in a combination of estrogen and progestins, also known as oral contraceptive pills. The adverse side effects of the oral contraceptive pills included cardiovascular diseases, venous risk and metabolic disorder like lipoprotein changes, altered insulin response to glucose and prolonged use of those even might lead to endometriosis [1-3]. The nonsteroidal anti fertility drugs e.g. Gonadotropin-releasing hormone analogues (goserelin, leuprolide triptorelin) that are marketed in place of steroidal contraceptive pills also have severe side effects, affecting the central nervous system, associated CNS depression [4]. Hence search of newer alternatives for female contraception from natural products is going on and is still a challenge to scientists’ globally [5,6].
Snake shed skin has long been used in the Chinese traditional medicine for various disorders including malignant sores, such as mammary abscess and tumor, boils, carbuncles, and furuncles. The shedded skins are usually roasted and then used both internally and topically. The snake shed skin considered useful in reducing clouding of the cornea. In Indian traditional, folk and Santhal medicinal system mentioned the use of snake shed skin for reproductive disorders, and previous studies had shown that Naja naja shedded skin influenced rat estrous cycle [7]. However no reports are available regarding the presence of any bioactive compounds in the N. naja shedded skin. The present study was designed to isolate bioactive molecule from N. naja shedded skin and the probable molecular action on female estrous cycle.
Materials and Methods
Chemical and reagentCarbinol and HPLC water were purchased from Spectrochem (India), IL-1β, TNF-α ELISA kits were purchased from R&D system (Minneapolis, USA), Hormones (Estradiol, Progesterone) were procured from Cusabio, China. Prestained molecular weight marker (Fermentas, USA), Primary ntibody (β-actin, Bax, BCL2, Caspase 3,8,9, PARP, HSP70, and HSP90) and alkaline phosphatase onjugated secondary antibodies were purchased from Santacruz CA (USA), Ripa Lysis Buffer (Sigma ldrich, USA). All other chemicals purchased are of AR grade or otherwise mentioned.
Animals and experimental design
Female Swiss albino mice 10-12 weeks old were purchased from approved animal breeders of Maulana Azad College, Kolkata; they were housed in polypropylene cages at temperature (26±2 °C) and light controlled environment (12 h light/dark). All the animals were fed with standard laboratory diet, had free access to food and water ad libidum. Estrous cycle was followed by carrying out vaginal smears daily. Vaginal lavage was performed using approximately 30 μl of 0.9% NaCl, dispensed in the vagina through plastic tip applicator. Cells were collected and studied under microscope to confirm each stage of estrous cycle. Animals showing two consecutive cycles were used for the experimental study. Regulations of the Animal Experimental Ethics Committee were followed, and were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA) [25/250/2012- AWD], Government of India. Vaginal lavage was observed every 24 h for 5 consecutive days.
Collection of N. naja shedded skin
Fresh Naja naja shedded skins of both sexes were collected from North 24-Parganas of West Bengal, India as per the permission granted by the Ministry of Forests & Wild Life, Govt. of West Bengal, India [2105/WL/4R-l (PI-IX)].
Isolation, purification and characterization of active fraction
Prior to TLC, 8 g N. naja shedded skin was dissolved in 2 ml HPLC water, kept overnight at 4 °C followed by centrifugation. The supernatant was separated and TLC was done on preactivated silica el G coated glass plates using isopropanol: 0.1 N HCl (7:3, v/v). Spots were visualised in 0.1% ninhydrin and Rf was calculated, same protocol was followed for preparative TLC. Zones were scraped and eluted in methanol followed by centrifugation. The supernatant was kept at room temperature for 18-24 h to obtain zone 2. Prior to RPHPLC zone 2 was washed in solvents (diethyl ether, ethyl acetate,chloroform, methanol), and was further purified by RP-HPLC (Jasco V 2080 HPLC system, C18 column 5 μm, 250 mmX 4.6 mm) usingmethanol: water (70: 30, v/v) and visualized at 254 nm for 90 min to obtain NNSS2. M.W of NNSS2 was confirmed through MALDI-TOF (4700 Linear Spectrophotometer). Secondary structure of the peptidewas confirmed by CD spectral analysis. The probable constituents and chemical nature were analysed by FTIR spectroscopy (Perkin Elmer RX-1 FTIR express version 1.03.00 spectrophotometer), H1NMR (AV 300 MHz NMR system dual probe) and x-ray diffraction (PAN analytical PW.3040/60, Netherland).
Effect of NNSS2 on serum hormones and cytokines level
On day 5 following NNSS2 exposure serum 17-β estradiol, progesterone, IL-1β and TNF-α levels were measured by ELISA.
Histopathological studies of NNSS2 treated mice uterus and ovary
On day 5 following NNSS2 exposure uterus and ovaries of mice were isolated and fixed in 10% buffered formalin followed by dehydration, clearing and paraffin embedding. 5 μ sections were stained with haematoxylin-eosin and observed under bright field microscope.
Western immunoblot analysis
Expressions of different proteins (Bax, BCl2, HSP 70, HSP 90, PARP, and β-actin) of uterus and ovary tissue homogenate were analysed by western blot. For preparation of tissue homogenate uterus and ovaries were isolated and placed in cold PBS (pH 7.2). 10% tissue homogenate was prepared in RIPA lysis buffer in the presence of protease inhibitor cocktail. After incubation period of 1 h at 4 °C, it was centrifuged at 12,000 rpm for 30 min (4 °C). The supernatant was collected and stored in -20 °C for future use. Equal concentration and volume of cellular protein samples were run in 12.5% SDS PAGE and transferred to nitrocellulose membrane in an immunoblot apparatus using transfer buffer (193 mM Glycine, 25 mM Tris and 20% methanol) and were blocked using 5% BSA in TBST (Tris buffered saline-Tween 20) for 2 h. The membrane was next incubated with respective primary antibody diluted with TBST containing 0.3% BSA and kept for overnight. Next day, the membrane was washed with TBS (Tris buffered saline) to remove unbound primary antibodies and next incubated with respective secondary antibody (alkaline phosphatase conjugated). The membrane was washed with TBS and colour was developed using NBT-BCIP mix in dark. Finally membranes were scanned for future documentation. After westernblot, each bands (except PARP, as it was cleaved after treatment) were analyzed through ImageJ software for quantitative measurement. In brief, a constant unit area was selected and average density of the total band area of the respective proteins in control and treated groups were measured and represented graphically
Statistical Analysis
The results were expressed in terms of mean± SEM (n=6). The data were subjected to one way analysis of variance (ANOVA) followed by Tukey’s test using GraphPad InStat 3 software to establish statistical significance (*p<0.05, **p< 0.01, ***p< 0.001).Results
Isolation, purification and characterization of NNSS2TLC of N. naja shedded skin aqueous extract showed four zones (Zone 1-Zone 4) having Rf 0.65, 0.77, 0.827 and 0.98 (Figure 1a). RPHPLC produced a single peak with retention time of 5.8 min and nomenclature as NNSS2 (Naja naja shedded skin zone 2) (Figure 1c). Spectral analysis of NNSS2 showed λmax at 287.2 nm (Figure 1d). MALDI-TOF analysis confirmed M.W of NNSS2 of 882.6 Dalton (Figure 1e). CD-spectral analysis of NNSS2 showed presence of negative bands between 217-229 nm, 236-331 nm, positive region between 230-235 nm, 196-215 nm. CD-spectral analysis showed 4% α-helix, 48% β sheet, 0.4% random coils and β-turn (Figure 2a). For FTIR and H1NMR see Table 1 and Figures 2b and 2c. X-ray crystallographic structural analysis showed presence of sharp peak which revealed that NNSS2 is a highly crystalline compound (Figure 2d).
Figure 1: Isolation, purification of NNSS2.(a) Thin layer chromatography of Naja naja shedded skin resolved into four zones using solvent system isopropanol: 0.1 N HCl (70:30, v/v); (b) Preparative thin layer chromatography using solvent isopropanol: 0.1 N HCl (70:30) was resolved into 4 zones (Zone 1 - Zone 4). Zone 2 was isolated and eluted in methanol; (c) RP-HPLC analysis of NNSS2 using solvent methanol:water (70:30) and C18 column produced retention time of 5.8 min; (d) Spectral analysis of NNSS2 showed λmax of 287.2 nm; (e) MALDI–TOF of NNSS2 showed M.W of 882.6 D.
Effect of NNSS2 on estrous cycle
NNSS2 (1 mg kg-1 body weight, s.c) produced cessation of the estrous cycle at the diestrous phase for 5±1 day when vaginal lavage was observed every 24 h for 5 days (Data not shown).
Effect of NNSS2 on serum hormones and cytokines
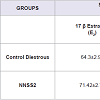
NNSS2 (1 mg kg-1 body weight, s.c) significantly decreased (p< 0.001) progesterone on Day 5 and no significant change was observed in estradiol (p>0.05) when compared to diestrous control adult female mice but it significantly decreased serum P4/E2 ratio (Table 2). NNSS2 (1 mg kg-1 body weight, s.c) caused a significant increase in the serum IL-1β (p< 0.001) and did not produce significant change in serum TNF-α (p>0.05) when compared with diestrous control adult female mice (Table 2).
Histopathological studies of NNSS2 treated mice uterus and ovary
NNSS2 (1 mg kg-1 body weight, s.c) treatment produced loss of normal uterine architecture, thinning of endometrial, myometrial, perimetrial lining, constriction of luminal structure, formation of vacuolated structure and loss of uterine glands (Figure 3a). NNSS2 (1 mg kg-1 body weight, s.c) treatment produced phase arrest in the follicle, showed distorted follicular structure, absence of matured follicle, ovum, granulosa and thecal cell lining as compared with control diestrous ovary (Figure 3b).Figure 3: Histopathological studies of NNSS2 treated ovary and uterus.Histological structure of control diestrous uterus (A) NNSS2 treated mice uterus (B) Histological structure of control diestrous ovary (C) NNSS2 treated ovary (D) Control diestrous uterus. a,b) presence of perimetrium, myometrium, endometrium, uterine glands c) Star shaped lumen. Control diestrous ovary d) follicles at different stage of development. NNSS2 treated uterus x) absence of uterine glands y) presence of vacuolated structure, NNSS2 treated ovary e) distorted follicle f) immature constricted follicle g) phase arrest of follicles. All the sections were stained with haematoxylin and eosin and magnification was 65X.
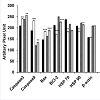
NNSS2 (1 mg kg-1 body weight, s.c) treated uterus and ovary showed up regulation of Caspase 3,9, Bax, and down regulation of BCL2 (Figure 4a). NNSS2 (1 mg kg-1 body weight, s.c) treated uterus and ovary showed PARP cleavage. NNSS2 (1 mg kg-1 body weight, s.c) treated uterus and ovary caused down regulation of heat shock proteins (HSP70 and HSP90) as compared with uterus and ovary from diestrous control mice (Figure 4a). Quantitative analysis through ImageJ software also confirmed significant up regulation of aspase 3, caspase 9, Bax and down regulation of BCL2, HSP70 and HSP90 expression significantly (Figure 4b).
We conclude that StnII/A10 gave exciting finding about the localization and the nature of the different lipid deposits in NPCderived cells that could improve the knowledge of lysosomal diseases.
Figure 4a: Western immunoblot analyses of intracellular apoptotic proteins.NNSS2 (1 mg kg-1 body weight) treated uterus and ovary. Protein from the total tissue lysate was subjected to SDS-PAGE and western immunoblot analysis was done using Caspase 3,9, Bax, BCL2, PARP, HSP70, HSP90, β-actin primary antibody. After primary antibody incubation, respective secondary antibodies were added and colour development was performed using NBT/BCIP mix. The relative intensity of each band was measured after normalization with the intensity of β-actin in a blot (shown below each western blot). Representative blots from three independent experiments gave identical results.UT: Uterus; OV: Ovary
Figure 4b: N4bN:S S2 (1 mg kg-1 body weight) treated uterus and ovary. Afterwestern blot analysis densitometry analysis of the respective protein bands were performed. Histograms showed representative pixel intensities (arbitrary units of densitometry analysis using ImageJ software) of the immunoblot. Values were represented as mean±SEM.
Discussion
This present study was designed to isolate a bioactive factor from Naja naja shedded skin. In the present study, a small molecular weight crystalline peptide NNSS2 of 882.6 Dalton was isolated from Naja naja shedded skin. CD spectral analysis indicated NNSS2 is composed of β sheet random coils and α helix. NNSS2 is crystalline and is composed of β-sheeted structure, N-H bonding, scissoring, bending and double bonds. Six amino acids had been identified so far namely asparagine, aspartate, glutamine, glycine, phenylalanine and tryptophan from.NNSS2 caused cessation of the estrous cycle at diestrous phase for five days when observed microscopically, with corresponding change in ovarian and uterine histology. NNSS2 caused loss of three distinct layers, uterine glands and luminal structure with formation of vacuolated structure (Figure 3B). NNSS2 produced constricted ovarian structure, follicular atresia, phase arrests, absence of granulosa cell lining (Figure 3D), indicating uterine and damage and may be the cause behind phase arrest. The histological findings of both ovary and uterus supported tissue damage suggesting anovulation and phase arrest at diestrous. Tissue damage that had taken place in the uterus and ovary might have occurred either by apoptosis or by necrosis. Hence, to further elucidate the death pathway, expression of intracellular apoptogenic proteins of ovarian and uterine tissues were studied. In both the ovary and uterus increased the expression of caspase 3 and caspase 9 and cleaved PARP with no change in caspase 8activities indicated involvement of intrinsic apoptotic pathway. PARP cleavage by caspase 3 is a considerable marker for apoptosis [8]. In the present study NNSS2 caused PARP cleavage indicating apoptosis through caspase 3 dependent pathway. No significant change in TNF-α level further confirmed absence of extrinsic pathway as TNF-α receptor is responsible for the activation of caspase 8 through the death receptor complex [9]. The intrinsic pathway of apoptosis gets activated by negative signal given by decreased level of progesterone and a positive signal from increasing expression of IL-1β, eventually activated the caspase dependent pathway [10]. This mitochondrial dependent pathway is regulated by BCL2 family of proteins which included proapoptotic protein like Bax and antiapoptotic protein like BCL2 by modifying mitochondrial membrane permeability. NNSS2 increased expression of proapoptotic protein Bax and decreased the expression of antiapoptotic protein BCL2, ultimately leading to the activation of effector molecule caspase 3. Activation of caspase 3 leadsto the proteolytic cleavage of PARP responsible for morphological and biochemical changes in apoptosis [11,12].
HSP70 and HSP90 are molecular chaperone proteins associated with intracellular stress and play a critical role in cytoprotection [13]. HSP70 blocks apoptosis by binding to Apaf-1, prevented the formation of apoptosomal complex further inhibiting caspase 3 induced proteolytic cleavage of PARP [14]. HSP90 another chaperone protein directly binds to Apaf-1 inhibited apoptosome complex formation [15]. In the present study NNSS2 down regulated the expression of both HSP70 and HSP90 indicating apoptosis induction, suggesting follicular atresia and estrous cycle arrest at diestrous phase.
In the serum NNSS2 significantly decreased progesterone (Table 1); progesterone secreted from the corpus luteum is associated with anti apoptotic activity and exerts direct effect on luteal action [16]. Other than antiapoptotic activity progesterone is also associated with granulosa cell development [17], steroidal bio synthesis and number of cell signalling activities and is also intricately associated with cytokine regulatory pathway [18,19]. Present study revealed that NNSS2 significantly increased serum IL-1β expression. It is a very well documented fact that increased expression of IL-1β is associated with the induction of apoptosis by the involvement of ICE which converts the pro enzyme in to its active form [20]. From the present study it was revealed that NNSS2 influenced the estrous cycle in female albino mice by causing cessation of the estrous cycle at diestrous phase for5 days.
Conclusion
NNSS2 caused anovulation hence it can lengthen a normal cycle by modulating the hormones, cytokines and intracellular apoptotic proteins. From the above findings it could be concluded that this study gave a novel insight into some of probable bioactive factors present in snake (N. naja) shed skin which remained unexplored until now.Acknowledgements
We thankfully acknowledge University Grant Commission, New Delhi, India for partial financial assistance (Ref No: F 38-130 (SR), dated 19.12.2009 and we are thankful to the Department of Forests & Wild Life, Government of West Bengal, India (2105/WL/4R-1(PI- IX)) for kind permission of collection of the shed snake skin.References
- Chapron C, Souza C, Borghese B, Lafay-Pillet MC, Santulli P, et al. (2011) Oral contraceptives and endometriosis: the past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum Reprod 26: 2028-2035.
- Sitruk-Ware R, Nath A (2011) Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord 12: 63-75.
- Sitruk-Ware R, Nath A (2013) Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract Res Clin Endocrinol Metab 27: 13-24.
- Piva F, Sterescu N, Zanisi M, Martini L (1969) Non-steroidal antifertility agents affecting brain mechanisms. Bull World Health Org 41: 275-288.
- Satyavati GV (1984) Indian plants and plant products with antifertility effect. Anc Sci Life 3: 193-202.
- Kumar D, Kumar A, Prakash O (2012) Potential antifertility agents from plants: a comprehensive review. J Ethnopharmacol 140: 1-32.
- Mukherjee S, Dasgupta SC, Gomes A (2013) Effect of Naja naja Laurenti shed skin extract on estrous cycle, hormone-cytokine profiles, histopathology of ovary and uterus of Swiss albino mice. Indian J Exp Biol 51: 235-240.
- Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, et al. (1999) Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274: 22932-22940.
- Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, et al. (2011) Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One 6: e28531.
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516.
- Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276: 7320-7326.
- Takahashi N, Tarumi W, Ishizuka B (2012) Acute reproductive toxicity of 3,3'-iminodipropionitrile in female rats. Reprod Toxicol 33: 27-34.
- Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther 101: 227-257.
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, et al. (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3: 839-843.
- Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula, SM, et al. (2000) Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 19: 4310-4322.
- Rueda BR, Hendry IR, Hendry III WJ, Stormshak F, Slayden OD, et al. (2000) Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biol Reprod 62: 269-276.
- Duffy DM, Stouffer RL (1995) Progesterone receptor messenger ribonucleic acid in the primate corpus luteum during the menstrual cycle: possible regulation by progesterone Endocrinol 136: 1869-1876.
- Peluso JJ, Pappalardo A (1994) Progesterone and cell-cell adhesion interact to regulate rat granulosa cell apoptosis. Biochem Cell Biol 72: 547-551.
- Vidaeff AC, Ramin SM, Gilstrap LC 3rd, Bishop KD, Alcorn JL (2007) Impact of progesterone on cytokine-stimulated nuclear factor-kappaB signalling in HeLa cells. J Matern Fetal Neonatal Med 20: 23-28.
- Friedlander RM, Gagliardini V, Rotello RJ, Yuan J (1996) Functional Role of Interleukin 1 beta (IL-1beta) in IL-1 beta-converting enzyme-mediated apoptosis. J Exp Med 184: 717-724.