Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: I. Michael Leitman, MD, Department of Surgery, Albert Einstein College of Medicine-Beth Israel Medical Center, Philips Ambulatory Care Center, 10 Union Square East, Suite 2M, New York, NY 10003, USA, Tel: 212-844-8570; Fax: 212-844-8440; E-mail: mleitman@chpnet.org
Citation: Bernstein L, Sabat JE, Montgomery M, Nwoke F, Natcheva H, et al. Postoperative Pneumoperitoneum: Clearing the Air. J Surgery. 2013;2(1): 4.
Copyright © 2013 Bernstein L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 2, Issue: 1
Submission: 28 October 2013 | Accepted: 06 November 2013 | Published: 11 November 2013
Reviewed & Approved by: Dr. Luigi Bonavina, Department of Surgery, University of Milan, School of Medicine, Italy
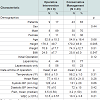
The vital signs, pain score, and leukocytosis were compared for each group on POD # 0. The data for both groups were similar with no statistically significant difference for any of these parameters. At the time of surgery, the same parameters were compared for each group. While the vital signs were the same for both groups at the time of imaging, there was a statistically significant difference found in the average white blood cell count of each group. In Group A, those patients requiring a reoperation had an average leukocytosis of 17.4 ± 12.0 versus 9.7 ± 4.9 in Group B (p = 0.01). Group A was also observed to have postoperative free air further out from surgery at an average postoperative day of 7.7 ± 2.6 versus 4.3 ± 2.9 in Group B (p = 0.003).
Postoperative Pneumoperitoneum: Clearing the Air
Laura Bernstein, Joseph E. Sabat, Marissa Montgomery, Franklin Nwoke, Hristina Natcheva, Anthony Sorrentino, Charles Holland, Barbara Zeifer, Sebastiano Cassaro, David Lucido, Burton Surick, and I. Michael Leitman*
- Department of Surgery, Albert Einstein College of Medicine – Beth Israel Medical Center, New York, NY, USA
*Address for Correspondence: I. Michael Leitman, MD, Department of Surgery, Albert Einstein College of Medicine-Beth Israel Medical Center, Philips Ambulatory Care Center, 10 Union Square East, Suite 2M, New York, NY 10003, USA, Tel: 212-844-8570; Fax: 212-844-8440; E-mail: mleitman@chpnet.org
Citation: Bernstein L, Sabat JE, Montgomery M, Nwoke F, Natcheva H, et al. Postoperative Pneumoperitoneum: Clearing the Air. J Surgery. 2013;2(1): 4.
Copyright © 2013 Bernstein L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 2, Issue: 1
Submission: 28 October 2013 | Accepted: 06 November 2013 | Published: 11 November 2013
Reviewed & Approved by: Dr. Luigi Bonavina, Department of Surgery, University of Milan, School of Medicine, Italy
Abstract
Introduction: Pneumoperitoneum seen on postoperative imaging presents a diagnostic dilemma. It can be a normal finding secondary to air that was introduced at surgery, which typically resolves in a matter of days. On the other hand, it could also represent a sign of a perforated viscus or an anastomotic leak, which might require reoperation. Distinguishing one from the other is critical to successful management. This study examines clinical and radiological findings in order to determine objective criteria to facilitate the distinction between benign and pathological postoperative pneumoperitoneum.Methods: A retrospective analysis of medical records from a large urban teaching hospital was performed. Imaging studies reporting “pneumoperitoneum”, “free air”, and “free intraperitoneal air”, from 2008-2011 were selected for review. The cases were divided into two groups: patients who ultimately were returned to the operating room and had findings requiring operative intervention and those who were managed expectantly. Demographic, physical findings and laboratory studies were recorded.
Results: 52 patients were found to have postoperative pneumoperitoneum after abdominal surgery. Nine (17.3%) underwent re-exploration because of presumed intra-abdominal complication and the remainder of patients was managed by observation alone. At re-operation, all 9 patients were found to have pathologic conditions requiring intervention.
Thirty-seven patients had an open surgery initially and 15 had a minimally invasive abdominal procedure. The patients in each group were similar with regard to age, gender, vital signs, pain score, physical findings, or open vs. laparoscopic procedure. However, patients requiring re-operation were found to have pneumoperitoneum 7.7 days after initial surgery compared to postoperative 4.3 days for those that could be managed expectantly (P = 0.003). Also, patients requiring re-operation had an average WBC of 17.4 compared to 9.7 for those managed conservatively. This suggests that postoperative pneumoperitoneum after postoperative day 5 with a WBC greater than 10.5 was 80 % sensitive for patients requiring re-operation.
Conclusion: This study suggests that patients with postoperative free air still present a diagnostic and therapeutic challenge. However, free air several days following surgery with an elevated WBC may provide an indication that this finding should be of greater concern. Such patients have a greater likelihood of requiring reoperation for the treatment of a postoperative complication.
Introduction
When a postoperative patient undergoes imaging that reveals postoperative pneumoperitoneum, it becomes a diagnostic dilemma to determine whether this intra-abdominal air is benign or pathologic (Figure 1). The ability to correctly distinguish between the two is paramount in the patient’s management in order to avoid the potentially unnecessary morbidity of a laparotomy or, on the other hand delaying a life-saving operation.Physiologic postoperative pneumoperitoneum represents either atmospheric air retained in the abdominal cavity after an open abdominal operation or residual insufflated gas used to provide visualization and increased working space during a laparoscopic operation. This physiologic pneumoperitoneum is observed after 60% of open and 25% of laparoscopic surgeries [1]. It is a benign finding that may be managed simply with observation of the patient. Physiologic postoperative pneumoperitoneum will resolve spontaneously with time, depending upon the imaging study used and certain patient characteristics. On plain chest or abdominal radiographs, it will resolve in 2 days in 67% of patients and in 5 days in 97% of patients [2]. On CT scan, however, it will still be seen at 3 days postoperatively in 85% of patients and 6 days postoperatively in 50% of patients [3]. This is because CT scan is more sensitive at detecting pneumoperitoneum than plain radiography. Body habitus has been shown to have an effect on the duration of postoperative pneumoperitoneum on plain radiographs, with obese patients showing faster resolution of air than asthenic patients. This is not the case when utilizing CT scans [4].
Pathologic postoperative pneumoperitoneum results from a complication that develops some time after surgery. Most commonly, it indicates a surgical complication resulting non-contained abscess or a break in the continuity of the gastrointestinal tract, from a perforated viscous or an anastomotic leak. These conditions require prompt recognition and intervention in order to minimize morbidity and mortality. Most times the patient must be urgently returned to the operating room for exploration and definitive treatment (Figure 2).
While some studies have investigated the phenomenon of physiologic postoperative pneumoperitoneum, very few have endeavored to explore pathologic postoperative pneumoperitoneum. In fact, Gayer et al. [4] concluded that the radiological demonstration of free air in itself should not play a major role in clinical decisionmaking. In this study, we sought to discover any variables associated with postoperative pneumoperitoneum that would help predict which postoperative patients will require emergent intervention. Specifically, how can a clinician reliably determine whether the air seen on postoperative imaging is benign or pathologic?
Methods
The Institutional Review Board at Beth Israel Medical Center approved this study. A search of our imaging database from 2008 to 2011 for CT, chest X-ray, and abdominal films containing the phrases “free air”, “free intraperitoneal air”, and “pneumoperitoneum” were identified. The corresponding charts for radiological studies with these findings in postoperative patients were collected and divided into two groups. In one group were patients who had postoperative free air and returned to the operating room, and in the second group were patients who also had postoperative free air, but did not return to the operating room. The charts were analyzed for the following: age, gender, height, weight, vital signs, pain score, and leukocytosis both at the time of surgery and the time of imaging. The types of imaging and postoperative day of the observed free air were also collected. Continuous variables were tested for statistical significance with Student’s t-test, proportions with chi-squared statistic, and discrete variables with the Mann-Whitney U-test. Logistic regression was used to calculate the odds ratio for the post op day and leukocytosis.Results
The results are shown in Table 1. Between 2008 and 2011 there were a total of 52 patients with postoperative free air, 9 of whom required an operative intervention in Group A, and 43 of whom were managed without returning to the operating room in Group B. The average age of groups A and B were 52.0 and 54.9 years respectively. As shown in the table, their height, weight and BMI were similar. There was only one female requiring reoperation in group A (11%) versus 30% in group B, but this was not statistically significant (p = 0.44). The proportion of patients undergoing open surgery was 44% for group A and 77% for group B (p = 0.12).Discussion
In the present study, we evaluated the clinical data of patients who were found to have pneumoperitoneum on imaging obtained after an abdominal operation in order to determine if any variable exists that might help to differentiate between benign versus pathological pneumoperitoneum. We examined 52 patient cases, 9 of which were intervened upon operatively after their imaging study, and 43 of which were observed and managed expectantly. All nine patients who were re-operated on suffered a postoperative complication that resulted in the presence of pneumoperitoneum seen on their imaging study, and all 43 patients who were observed were managed successfully in an expectant manner.Based upon the data collected during our study, there appears to be an increased risk of postoperative pneumoperitoneum being pathologic in etiology when it is seen on postoperative day 6 or later, and when the patient has an elevated white blood cell count. Specifically, of the patients with postoperative pneumoperitoneum requiring re-operative intervention, 7/9 (78%) had an elevated white blood cell count and 8/9 (89%) were 6 days postoperative or later when the imaging study showing pneumoperitoneum was obtained. Additionally, of the patients managed expectantly, 40/43 (93%) had white blood cell counts within normal limits and were less than 7 days postoperative when imaging showed pneumoperitoneum.
Based upon the results of our study, we determined that the presence of postoperative pneumoperitoneum on imaging after postoperative day 5 in a patient with a white blood cell count > 10.5 is 80% sensitive for predicting that a patient will require operative intervention for pathologic pneumoperitoneum. This is the first study to correlate either postoperative date or presence and degree of leukocytosis as a risk factor for the need for operative intervention in patients with postoperative pneumoperitoneum. While we did not observe any negative re-laparotomies, using these criteria of WBC and postoperative day of observed pneumoperitoneum alone would have led to a false positive (type-I error) rate of 25%. While this may seem high, the morbidity and risks associated with a laparotomy are not insignificant. In one study by Ruler et al. [5], patients without clear indications for re-laparotomy (e.g. closed loops, intestinal ischemia, or patients with packing to control bleeding) were studied in order to find variables associated with a requirement for re-laparotomy. Of 219 patients, 117 underwent re-laparotomy, 55 of whom did not have positive findings at re-laparotomy (47%). Using various clinical data (but not imaging or pneumoperitoneum) they developed a model with similar sensitivity and false-positive rates as ours (82% sensitive with a false positive rate of 24%). Our data shows that postoperative pneumoperitoneum can be a criterion to consider when deciding on re-laparotomy.
Others have investigated pathologic postoperative pneumoperitoneum. Lee et al. [6] recently published a study looking at the duration of physiologic postoperative pneumoperitoneum on plain radiographs and the radiographic characteristics of pathologic postoperative pneumoperitoneum in patients with anastomotic leak. They found that the initial air height measured on erect abdominal radiograph in those with the anastomotic leaks was significantly greater than those with physiologic postoperative pneumoperitoneum (12.16 ± 7.65 mm vs. 7.71 ± 5.08 mm, P = 0.04). Through further analysis, they determined that an initial air height of >11.7 mm on erect abdominal radiograph, air height that increases with time, and presence of a postoperative ileus might be indicators of anastomotic leakage. Spinelli et al. [7] published a series of case reports of patients with postoperative pneumoperitoneum in order to create an algorithm for the management of postoperative pneumoperitoneum. They suggest operative exploration in patients with postoperative pneumoperitoneum on plain radiograph in the presence of peritoneal signs on abdominal exam, hemodynamic instability, signs of sepsis, or failure of initial conservative management. Additionally, they recommend obtaining a CT scan if there is suspicion of a postoperative complication. These are logical recommendations; however, they are based mostly upon the existing literature. Of their four cases, only one patient was taken to the operating room for exploration, and was negative for any intra-abdominal pathology.
Most studies examining postoperative pneumoperitoneum focus instead on physiological postoperative pneumoperitoneum. Shatari et al. [8] examined the incidence and duration of postoperative pneumoperitoneum identified on chest radiographs after open colorectal operations. Their results showed air with a height of < 15 mm in 15% of radiographs taken during postoperative days 0-5, 18% of during postoperative days 5-10, and none after postoperative day 10. Hope et al. [9] recently published a series on the incidence and duration of non-pathologic postoperative pneumoperitoneum after laparoscopic nephrectomy. Their results showed an incidence of postoperative pneumoperitoneum of 41% after laparoscopic nephrectomy. Most patients showed resolution of their pneumoperitoneum by postoperative day 3, however, it was seen up to nine days postoperatively in one patient. They maintain that their study population is unique in that the nephrectomy procedure entails an extensive retroperitoneal dissection.
Our study has several important limitations. The main weaknesses are the retrospective nature of the review and that the imaging studies were obtained for a variety of indications in a non-standardized manner. There were no studies done immediately postoperatively that would have allowed us to obtain our own incidence data and would have served as a baseline for comparison of studies obtained at a later date. Clearly, a larger prospectively performed study with standardized procurement of imaging would be the ideal way to confirm and expound upon the findings of this study. Finally, we are not able to definitively state that those patients who had pneumoperitoneum managed conservatively did not in fact suffer a postoperative complication. It is possible that a few of the patients with “benign” pneumoperitoneum actually had a pathologic or other process develop that turned out to be clinically benign and was treated successfully with conservative management. This entity has been named nonsurgical free air, and is defined by the fact that whatever the cause, it can be successfully managed in an expectant manner, or in some cases leads to a negative operative exploration [10]. We feel that the possibility of nonsurgical free air being present in our study patients does not significantly alter the utility of the results of the study, however, which is to aid in the prompt identification of those patients with pathologic postoperative pneumoperitoneum that requires emergent surgical intervention. Despite these limitations, though, we were able to make two interesting and potentially useful observations that can help guide clinicians when presented with the conundrum of postoperative pneumoperitoneum.
References
- Schauer PR, Page CP, Ghiatas AA, Miller JE, Schwesinger WH, et al. (1997) Incidence and significance of subdiaphragmatic air following laparoscopic cholecystectomy. Am Surg 63: 132-136.
- Nielsen KT, Lund L, Larsen LP, Knudsen P (1997) Duration of postoperative pneumoperitoneum. Eur J Surg 163: 501-503.
- Stapakis JC, Thickman D (1992) Diagnosis of pneumoperitoneum: abdominal CT vs. upright chest film. J Comput Assist Tomogr 16: 713-716.
- Gayer G, Hertz M, Zissin R (2004) Postoperative pneumoperitoneum: prevalence, duration, and possible significance. Semin Ultrasound CT MR 25: 286-289.
- van Ruler O, Lamme B, Gouma DJ, Reitsma JB, Boermeester MA (2007) Variables associated with positive findings at relaparotomy in patients with secondary peritonitis. Crit Care Med 35: 468-476.
- Lee CH, Kim JH, Lee MR (2012) Postoperative pneumoperitoneum: guilty or not guilty? J Korean Surg Soc 82: 227-231.
- Spinelli N, Nfonsam V, Marcet J, Velanovich V, Frattini JC (2012) Postoperative pneumoperitoneum after colorectal surgery: Expectant vs surgical management. World J Gastrointest Surg 4: 152-156.
- Shatari T, Clark MA, Keighley MR (2004) Duration of pneumoperitoneum on chest radiograph after open colorectal surgery. Tech Coloproctol 8: 27-30.
- Hope WW, Heniford BT, Norton HJ, Lincourt AE, Teigland CM, et al. (2009) Duration and clinical significance of radiographically detected “free air” after laparoscopic nephrectomy. Surg Laparosc Endosc Percutan Tech 19: 415-418.
- Mularski RA, Sippel JM, Osborne ML (2000) Pneumoperitoneum: a review of nonsurgical causes. Crit Care Med 28: 2638-2644.
- Alley JB, Corneille MG, Stewart RM, Dent DL (2007) Pneumoperitoneum after percutaneous endoscopic gastrostomy in patients in the intensive care unit. Am Surg 73: 765-767.