Journal of Surgery
Download PDF
Review Article
*Address for Correspondence: Hui B. Sun, Department of Orthopaedic Surgery and Radiation Oncology, Albert Einstein College of Medicine and Montefiore Medical Center, 1300 Morris Park Avenue, Golding 101, Bronx, NY 10461, USA, Tel: 718-430-4291; E-mail: herb.sun@einstein.yu.edu
Citation: Leong DJ, Zhang H, Xu L, Tang J, Hirsh DM, et al. Therapeutic Ultrasound: Osteoarthritis Symptom-Modification and Potential for Disease Modification. J Surgery. 2013;1(2): 5.
Copyright © 2013 Leong DJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 21 August 2013 | Accepted: 25 September 2013 | Published: 30 September 2013
Therapeutic Ultrasound: Osteoarthritis Symptom-Modification and Potential for Disease Modification
Daniel J. Leong1,2,3, Huagang Zhang2, Lin Xu1,2, Justin Tang2, David M. Hirsh1, John A. Hardin1, Luis Cardoso3, Chandan Guha2, Neil J. Cobelli1, and Hui B. Sun1,2*
- 1Department of Orthopaedic Surgery, Albert Einstein College of Medicine and Montefiore Medical Center, New York, USA
- 2Department of Radiation Oncology, Albert Einstein College of Medicine and Montefiore Medical Center, New York, USA
- 3Department of Biomedical Engineering, The City College of New York, New York, USA
*Address for Correspondence: Hui B. Sun, Department of Orthopaedic Surgery and Radiation Oncology, Albert Einstein College of Medicine and Montefiore Medical Center, 1300 Morris Park Avenue, Golding 101, Bronx, NY 10461, USA, Tel: 718-430-4291; E-mail: herb.sun@einstein.yu.edu
Citation: Leong DJ, Zhang H, Xu L, Tang J, Hirsh DM, et al. Therapeutic Ultrasound: Osteoarthritis Symptom-Modification and Potential for Disease Modification. J Surgery. 2013;1(2): 5.
Copyright © 2013 Leong DJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 21 August 2013 | Accepted: 25 September 2013 | Published: 30 September 2013
Abstract
Osteoarthritis (OA) is a degenerative joint disease and a leading cause of adult disability. While joint replacement surgery is a common treatment option for end-stage disease, non-surgical management is critical for preventing disability and maintaining quality of life. Although therapeutic ultrasound, which applies mechanical and may also apply thermal energy in the form of sound waves, is widely used to treat various musculoskeletal disorders such as bone fractures, tendinopathy, and muscle contusions, its symptom- and disease-modifying effects on osteoarthritis have not been clearly demonstrated. Recent clinical evidence indicates therapeutic ultrasound is capable of relieving OAassociated pain and improving function of diseased joints. Furthermore, in vitro and in vivo studies are beginning to emerge which suggest ultrasound may exert chondroprotection, such as enhancing anabolic activity, suppressing proteolytic enzyme-mediated degradation of the cartilage matrix, preventing chondrocyte apoptosis and modifying the endocrinology of adipose tissue that may potentially contribute to OA disease initiation and progression. Therefore, ultrasound may have great potential to serve as an effective and non-invasive therapeutic treatment for osteoarthritis.Keywords
Therapeutic ultrasound; Low intensity pulsed ultrasound; Osteoarthritis; Disease-modification; OA-associated painIntroduction
Osteoarthritis (OA) affects over 27 million Americans, is a leading cause of pain and disability [1,2], and is a significant economic burden in the United States with over $185.5 billion in annual medical care expenditures [3]. While OA is a disease of the entire synovial joint, and affects the underlying bone, synovium, meniscus, ligaments/tendons, and articular cartilage [4,5], erosion of articular cartilage is the pathological hallmark of osteoarthritis, and cartilage is a major target for exploring disease-modifying treatment [4,6-8]. Cartilage lines the ends of the bones, allowing for the articulation of opposing joint surfaces. Destruction of articular cartilage leads to bone-on-bone contact, causing stiffness, pain, and ultimately, loss of movement in the joints [9].There is currently no cure for OA. Therapies, which mainly address OA-related symptoms such as pain and dysfunction, have no demonstrated effect on slowing or arresting its progression [6,10]. End-stage disease often requires surgical intervention such as a total joint replacement. At earlier stages of OA, however, non-surgical management is critical for preventing disability and maintaining quality of life. Non-pharmacologic interventions, including mechanical-based therapies, are commonly recommended to OA patients [11].
Recent clinical trials show therapeutic ultrasound such as low-intensity ultrasound can improve OA-associated pain and dysfunction [12], although its effects in modifying disease progression require to be further studied. In this review, we will first provide a brief overview regarding the concept of therapeutic ultrasound and its current use in musculoskeletal tissue repair and disorders. We will then discuss recent clinical evidence of ultrasound in modifying OA-associated symptoms and mechanisms-based evidence that supports the concept of using ultrasound in chondroprotection and OA treatment.
Therapeutic Ultrasound and its Use in Musculoskeletal Tissue Repair and Disorders
Therapeutic ultrasound treatment, such as those using lowintensity ultrasound wave energy, are widely used to treat pain and various musculoskeletal disorders including bone fractures, shoulder pain, pressure ulcers, and muscle soreness [13]. Upon penetrating the biological tissue, these low-intensity ultrasound waves generate acoustic vibrations that cause local movement of cell membrane, fluid and macromolecules [14]. This produces mechanical stimulation that subsequently changes the physical and biological properties of the cells, such as cell membrane permeability, fluid movement and exchange of intracellular and extracellular ions, all of which eventually alter cell growth and metabolism [15].The actual biological effect of ultrasound therapy varies with the energy that is delivered to the tissue. The energy of ultrasound is expressed as sonic intensity (SI: W/cm2) that is proportional to sonic pressure square. Low-intensity ultrasound uses ultrasound with intensities less than 3 W/cm2 and is usually used as physiotherapy to stimulate cell proliferation and tissue repair [15]. On the contrary, high-intensity ultrasound approaches use focused ultrasound probes that concentrate the wave energy in a smaller tissue region, reaching intensities higher than 5 W/cm2, which can cause coagulative necrosis of tissues due to thermal absorption, and is normally used as an ablative agent to destroy target tissues [16]. Depending on the energy and way the ultrasound is delivered, the biophysical effects of ultrasound are traditionally separated into thermal and nonthermal effects. Thermal effects are caused by vibration or rotation of macromolecules in the tissue, which result in frictional heat and a rise in temperature. Non-thermal effects are characterized by the formation of tiny gas bubbles (stable cavitation) and the movement of liquid around the vibrating bubbles (acoustic streaming) in the tissue. Heat increases are predominately observed in tissues exposed to continuous high intensity ultrasound. In tissues treated with lowintensity pulsed ultrasound (LIPUS), the non-thermal effects are dominant [15].
The most common use of therapeutic ultrasound is for the facilitation of bone fracture healing. In 1983, LIPUS was found to heal 70% of non-unions in patients with lower extremity fractures [17]. Eleven years later, a randomized double-blinded controlled study was conducted by Heckman et al. [18]. Among 67 patients with closed or grade-I open fractures of the tibial shaft, 33 received LIPUS treatment and the average healing time in these patients was significantly decreased when compared with controls (86 vs. 114 days). Consistent with these results, another multicenter, prospective, randomized, double-blind and placebo-controlled clinical trial revealed significant acceleration of dorsal radius fracture healing in patients treated with LIPUS (61 vs. 98 days) [19]. In a large-scale efficacy assessment, successful healing rates of LIPUS in the treatment of delayed unions and non-unions were 91% and 86%, respectively [20].
Ultrasound is also used to treat tendon conditions such as tendinopathy and tendonitis [21,22]. In vitro studies demonstrate ultrasound enhances the proliferation and migration of tendon-reparative cells, and collagen synthesis in tendon cells, suggesting it may improve tendon healing [23]. In animal models of Achilles tendon rupture, daily ultrasound accelerated the healing process [24], and improved collagen alignment and mechanical strength in healing tendons compared to untreated controls [25]. However, clinical trials so far have not clearly demonstrated that therapeutic ultrasound improves treatment outcomes in tendon conditions such as patellar tendinopathy [22], but may accelerate the initial phase of the tendonbone healing process after rotator cuff repair [26].
Experimental evidence supporting the use of therapeutic ultrasound for skeletal muscle contusions is mixed. Ultrasound pulses (1.5 W/cm2, 20% duty cycle, 3 MHz frequency) applied to 12 adult female Sprague-Dawley rats with experimental right calf contusion injury resulted in significant satellite cell proliferation in the early phase of muscle regeneration [27]. However, the overall effect of ultrasound therapy on muscle regeneration was not significant due to unaffected recapillarization and myotube production. Studies by Karnes et al. revealed that continuous ultrasound therapy improved force production of injured muscle 7 days after injury [28]. Two subsequent randomized controlled trials of 100 male Wistar rats with contusion muscle injury found no evidence to support the effect of ultrasound therapy on muscle regeneration [29,30]. However, one recent study suggests LIPUS can enhance the regeneration of myofibers in both in vitro and in vivo muscle laceration models [31]. While these findings suggest ultrasound could improve muscle injury outcomes, more studies are needed to evaluate its therapeutic efficacies.
OA-Symptom Modification
Joint pain and dysfunction are two major symptoms that OA patients experience. A systemic review with meta-analysis compared outcomes including joint pain and function in six controlled trials where OA patients received ultrasound or a sham treatment [12]. In all six studies, ultrasound was applied at a 1 MHz frequency, but with varying intensities and dosing schedules. Overall, low-intensity pulsed ultrasound at doses < 150 Joules/cm2 significantly reduced patient-reported pain using a Visual Analog Scale (VAS) [Standard mean difference (Confidence Interval) = -0.49 (-0.79, -0.18), P = 0.002]. Self-reported function with the Lequesne index or WOMAC (Western Ontario and McMaster Universities Arthritis Index) score showed ultrasound intervention generally led to improvements in function, although differences were not statistically significant. Two of these studies monitored adverse events, and both reported no major complications. This review [12] concluded that ultrasound appears to be effective in decreasing OA-associated pain, and may improve function in patients with knee OA. However, more adequately powered and higher-quality clinical trials are needed to further confirm these conclusions.Subsequent clinical trials assessing the efficacy of low-intensity ultrasound intervention on OA have come to similar conclusions. A small trial was conducted with 12 OA patients who had been diagnosed with OA for an average of 5 years [32]. Continuous ultrasonic waves (1 MHz frequency, 0.8 W/cm2 power with a 5 cm diameter applicator) were applied to the medial and lateral parts of the knee for 3-4 minutes, 2 days/week, for 12 weeks. Patients reported reduced disability, according to the WOMAC scores (decrease from 53.5 ± 12.2 to 28.8 ± 14.8, P=0.0002), and improved function, as assessed by a six-minute walking test, after the ultrasound intervention (mean improvement of 14.1 ± 22.5%, P=0.04), when compared to assessments taken before ultrasound treatment [32].
Yang et al. conducted a clinical trial involving 100 OA patients, who had been diagnosed with OA for an average of 2.8 years, and subjected them to ultrasound or mock treatment [33]. The ultrasound treatment (parameters were not reported) consisted of 15 minutes of ultrasound application with three applicators which simultaneously stimulated the lateral and medial compartments, and medial joint space. Following 5 days of treatment, patients in the ultrasound group reported lower VAS and Lequesne scores (VAS efficacy index, mean = 0.3640, SD =0.28062, P = 0.000; Lequesne efficacy index, mean = 0.3080, SD = 0.42076, P = 0.000) [33].
A randomized, placebo-controlled double-blind study investigated the short-term efficacy of ultrasound therapy in 90 OA patients [34]. Patients were randomly assigned to three groups: continuous ultrasound (1 Mhz frequency and 2 W/cm2 power with a 5 cm diameter applicator) for 5 minutes, pulsed ultrasound (1 Mhz frequency and 2 W/cm2 power with a pulsed mode duty cycle of 1:4) for 5 minutes, or sham treatment for 5 minutes. Treatments were applied once a day, 5 days/week for 2 weeks. At the end of the study, patients in the pulsed ultrasound group showed the greatest reduction in pain (from 6.89 ± 1.39 to 5.25 ± 1.90, VAS score, p < 0.05) and WOMAC score (from 43.43 ± 8.26 to 35.61 ± 8.73, p < 0.05). Furthermore, walking time in a 20 meter test was shortened most significantly in the pulsed ultrasound group (from 22.57 ± 2.08 to 20.00 ± 1.94 seconds, p < 0.05) [34].
The mechanisms mediating the symptom-modifying effects of ultrasound on OA are not well established, largely because processes linking pain with OA are not well understood [35]. Of notice, recent evidence shows pro-inflammatory cytokines promote pain in OA by interacting with other biological mediators [36]. For example, pro-inflammatory cytokine interleukin (IL)-1β has been reported to stimulate nociceptors directly through intracellular kinase activation, and indirectly through the production of pro-inflammatory mediators including prostanoids [37]. Tumor necrosis factor (TNF)-α also has been demonstrated to activate sensory neurons directly [37,38], and anti-TNF-α treatment reduced OA-associated pain symptoms [39]. These mechanisms are of interest because LIPUS has been reported to reduce the inflammatory activity of synovitis in vivo, which was associated with a decrease in the number of cells expressing proinflammatory mediator cyclooxygenase 2 (COX-2) [40]. In vitro, LIPUS reduced levels of IL-1 and TNF-α in rat Schwann cells [41]. Together, these studies suggest therapeutic ultrasound may alleviate OA-associated pain by reducing inflammatory activity.
Potential for OA-Disease Modification
While recent clinical studies provide evidence that supports ultrasound exerting OA-symptom modifying effects, it is not clear whether ultrasound exerts effects on disease-modification, such as arresting or slowing OA disease progression. Interestingly, recent preliminary evidence suggests ultrasound may be used for chondroprotection by enhancing anabolic activity, suppressing catabolic activity, preventing chondrocyte apoptosis, and altering obesity-related inflammatory metabolism.The anabolic effect of ultrasound has been previously demonstrated in small animal studies. In New Zealand rabbits with full-thickness osteochondral defects, daily LIPUS treatment significantly improved the morphologic features and histologic characteristics of the repaired cartilage [42,43]. Subsequent studies in a canine model further demonstrated a positive effect of LIPUS treatment on cartilage repair [44]. In an in vitro 3D agarose gel culture model, LIPUS stimulated aggrecan and type II collagen synthesis but did not affect the proliferation of human chondrocytes [45]. Results from an in vitro 3D alginate bead model showed LIPUS increased the number and size of glycosaminoglycan-positive lacunae and cellular organelles in human chondrocytes [46].
Ultrasound has also been reported to reduce catabolic activity in chondrocytes. In OA joints, proteolytic enzymes, such as matrix metalloproteinases (MMPs)-1, -3, -13, and ADAMTS (a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs), are overactivated. These enzymes directly cleave the cartilage matrix, leading to a homeostatic imbalance and cartilage breakdown [47-49]. Ito et al. found in vitro, LIPUS (0 to 120 mW/cm2) reduced MMP-13 expression in an intensity dependent manner, with the greatest decrease seen at 120 mW/cm2 [50]. The authors also reported LIPUS downregulated expression of MMP-3 and MMP-13 in porcine cartilage explants [50]. Li et al. assessed the efficacy of LIPUS on preventing OA in a surgically-induced model (transaction of the anterior cruciate ligament) in rabbits [51]. Immediately after surgery, animals were treated with LIPUS at 3 MHz, 20% duty cycle, 40 mW/cm2 for 20 minutes/day, 6 days/week, for 6 weeks. Sham-treated animals were handled in the same manner as the LIPUS group, but not subject to ultrasound. At six-weeks following treatment, it appears that LIPUS exerted an OA disease modification effect, because LIPUS-treated animals had a significantly lower histopathological cartilagescore compared to sham-treated animals (sham treatment: 10.33 ± 2.66, ultrasound treatment: 6.67 ± 1.21, P < 0.05, Mankin grading system). Consistent with this observation, a reduced level of MMP-13 was also observed in the cartilage of LIPUS-treated animals [51].
In osteoarthritis, the fate and function of chondrocytes is altered, as evidenced by their abnormal proliferation, senescence, and cell death [4,52]. In a study to determine whether ultrasound can be used to prevent chondrocyte apoptosis in OA, OA was first surgically-induced in rabbits using the anterior cruciate ligament transaction model [53]. For the experiment, LIPUS was applied at six weeks following surgery, at an intensity of 300 mW/cm2 at 1 MHz, 20% duty cycle for 10 minutes/day for 2 weeks. At the end of the ultrasound treatment, microscopic morphologic grading showed the ultrasoundtreated group had a significantly lower OA score compared to untreated controls (control: 2.75 ± 0.50, ultrasound-treated: 1.67 ± 0.52, P=0.002). There was also a trend for a lower percentage of apoptotic chondrocytes in animals treated with LIPUS, although the difference was not significant [53].
Adipose Modification and Chondroprotection
Obesity is one of the risk factors for OA initiation and disease progression [54]. Studies suggest that obesity contributes to OA through mechanical overloading and metabolic alteration [55]. Excessive adipose tissue increases mechanical stresses on weight-bearing joints and generates an imbalance in the secretory profile of adipokines, including leptin, adiponectin, visfatin, and resistin [56]. Together, such conditions create a low-grade systemic inflammation, as evidenced by a significant increase, as much as 10-fold, in the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α [57,58]. These pro-inflammatory cytokines can then in turn upregulate expression of MMPs and ADAMTS, leading to cartilage breakdown [59].Randomized controlled clinical trials show weight loss is associated with reductions in knee OA pain, increased mobility and physical function [60,61]. Evidence shows each pound of weight lost results in a 4-fold reduction in the compressive forces through the load-bearing joints [62]; losing less than 5% body weight results in some joint pain relief, while moderate to large clinical improvements in joint pain are observed with at least 10% reductions in body weight [63].
Although the efficacy of ultrasound in osteoarthritis has not been studied in the context of obesity, recent studies suggest highintensity focused ultrasound (HIFU) is an effective method for breaking down fat cells [64-66]. HIFU is delivered through the skin and ultrasound energy absorption within the focal zone induces high temperatures at the focal point, causing coagulative necrosis and almost instantaneous cell death [67]. After the treated adipose tissue is destroyed, chemotatic signals activate the body’s normal inflammatory response mechanisms. Macrophage cells engulf the lipids and cellular debris, and they are cleared via the lymphatic system, leading to a reduction in adipose tissue [64]. Taken together, by targeting adipose tissue, ultrasound may exert chondroprotection by both directly reducing mechanical overloading stress, and rebalancing the altered inflammatory metabolism.
Perspectives and Conclusion
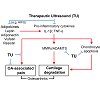
Therapeutic ultrasound is widely used for various musculoskeletal disorders, but its use for osteoarthritis treatment is still limited. Recent clinical trials suggest ultrasound improves OA-associated symptoms, including pain and joint dysfunction. However, well-designed and higher powered clinical studies are needed to confirm these effects. Furthermore, while disease-modifying effects of ultrasound have not been reported in OA patients, supportive data from in vitro and in vivo studies suggest a chondroprotective role of ultrasound, which includes enhancing anabolic activity, lowering levels of catabolic activity, and preventing apoptosis in chondrocytes. In addition, adipose tissue, which creates an inflammatory endocrine environment and may be a driver of OA initiation and progression, can be targeted by ultrasound (e.g. high-intensity focused ultrasound). Collectively, we propose ultrasound as a potential intervention for OA symptom- and disease-modification (Figure 1). In summary, therapeutic ultrasound may exert effects not only on symptom-modification but also has a strong potential for chondroprotection and disease-modification in OA. A better understanding of the mechanistic actions of ultrasound may transform ultrasound into a highly effective, non-invasive modality for osteoarthritis prevention and treatment.Figure 1: Ultrasound as a potential intervention for OA symptom- and disease-modification. Recent clinical evidence suggests therapeutic ultrasound (TU) relieves OA-associated pain and improves function, which may be mediated by the anti-inflammatory effects of ultrasound. In vitro and in vivo studies show therapeutic ultrasound reduces catabolic activity and apoptosis in chondrocytes, and high-intensity ultrasound (HIFU) ablates fat tissue and modulates adipokines, which together, may exert disease-modifying effects in OA.
References
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, et al. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58: 26-35.
- Suri P, Morgenroth DC, Hunter DJ (2012) Epidemiology of osteoarthritis and associated comorbidities. PM R 4: S10-S19.
- Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA (2009) Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum 60: 3546-3553.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64: 1697-707.
- Burr DB, Gallant MA (2012) Bone remodelling in osteoarthritis. Nat Rev Rheumatol 8: 665-673.
- Le Graverand-Gastineau MP (2010) Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr Drug Targets 11: 528-535.
- Evans CH, Ghivizzani SC, Robbins PD (2011) Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol 7: 244-249.
- Heinegard D, Saxne T (2011) The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol 7: 50-56.
- Sun HB (2010) Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci 1211: 37-50.
- Hashimoto M, Nakasa T, Hikata T, Asahara H (2008) Molecular network of cartilage homeostasis and osteoarthritis. Med Res Rev 28: 464-481.
- Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, et al. (2013) EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 72: 1125-1135.
- Loyola-Sanchez A, Richardson J, MacIntyre NJ (2010) Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartilage 18: 1117-1126.
- Robertson VJ, Baker KG (2001) A review of therapeutic ultrasound: effectiveness studies. Phys Ther 81: 1339-1350.
- Kim YS, Rhim H, Choi MJ, Lim HK, Choi D (2008) High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol 9: 291-302.
- ter Haar G (2007) Therapeutic applications of ultrasound. Prog Biophys Mol Biol 93: 111-129.
- Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5: 321-327.
- Xavier Cam DL (1983) The stimulation of bone callus by ultrasound. Rev Rras Ortop 18: 73-80.
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF (1994) Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 76: 26-34.
- Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR (1997) Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 79: 961-973.
- Mayr E, Frankel V, Ruter A (2000) Ultrasound--an alternative healing method for nonunions? Arch Orthop Trauma Surg 120: 1-8.
- Adahan HM, Sharon H, Siev-Ner I (2010) A sound solution to tendonitis: healing tendon tears with a novel low-intensity, low-frequency surface acoustic ultrasound patch. PM R 2: 685-687.
- Larsson ME, Kall I, Nilsson-Helander K (2012) Treatment of patellar tendinopathy--a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 20: 1632-1646.
- Tsai WC, Tang ST, Liang FC (2011) Effect of therapeutic ultrasound on tendons. Am J Phys Med Rehabil 90: 1068-1073.
- Ng CO, Ng GY, See EK, Leung MC (2003) Therapeutic ultrasound improves strength of achilles tendon repair in rats. Ultrasound Med Biol 29: 1501-1506.
- Fu SC, Shum WT, Hung LK, Wong MW, Qin L, et al. (2008) Low-intensity pulsed ultrasound on tendon healing: a study of the effect of treatment duration and treatment initiation. Am J Sports Med 36: 1742-1749.
- Lovric V, Ledger M, Goldberg J, Harper W, Bertollo N, et al. (2013) The effects of low-intensity pulsed ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Sports Traumatol Arthrosc 21: 466-475.
- Rantanen J, Thorsson O, Wollmer P, Hurme T, Kalimo H (1999) Effects of therapeutic ultrasound on the regeneration of skeletal myofibers after experimental muscle injury. Am J Sports Med 27: 54-59.
- Karnes JL, Burton HW (2002) Continuous therapeutic ultrasound accelerates repair of contraction-induced skeletal muscle damage in rats. Arch Phys Med Rehabil 83: 1-4.
- Markert CD, Merrick MA, Kirby TE, Devor ST (2005) Nonthermal ultrasound and exercise in skeletal muscle regeneration. Arch Phy Med Rehabil 86: 1304-1310.
- Wilkin LD, Merrick MA, Kirby TE, Devor ST (2004) Influence of therapeutic ultrasound on skeletal muscle regeneration following blunt contusion. Int J Sports Med 25: 73-77.
- Chan YS, Hsu KY, Kuo CH, Lee SD, Chen SC, et al. (2010) Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: an in vitro and in vivo study. Ultrasound Med Biol 36: 743-751.
- Mascarin NC, Vancini RL, Andrade ML, Magalhaes Ede P, de Lira CA, et al. (2012) Effects of kinesiotherapy, ultrasound and electrotherapy in management of bilateral knee osteoarthritis: prospective clinical trial. BMC Musculoskelet Disord 13: 182.
- Yang PF, Li D, Zhang SM, Wu Q, Tang J, et al. (2011) Efficacy of ultrasound in the treatment of osteoarthritis of the knee. Orthop Surg 3: 181-187.
- Tascioglu F, Kuzgun S, Armagan O, Ogutler G (2010) Short-term effectiveness of ultrasound therapy in knee osteoarthritis. J Int Med Res. 38: 1233-1242.
- Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, et al. (2013) A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527: 440-447.
- Schaible HG, Ebersberger A, Natura G (2011) Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther 13: 210.
- Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361: 184-187.
- Aoki Y, Ohtori S, Ino H, Douya H, Ozawa T, et al. (2004) Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine (Phila Pa 1976) 29: 2621-2626.
- Grunke M, Schulze-Koops H (2006) Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis 65: 555-556.
- Nakamura T, Fujihara S, Yamamoto-Nagata K, Katsura T, Inubushi T, et al. (2011) Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng 39: 2964-2971.
- Tsuang YH, Liao LW, Chao YH, Sun JS, Cheng CK, et al. (2011) Effects of low intensity pulsed ultrasound on rat Schwann cells metabolism. Artif Organs 35: 373-383.
- Cook SD, Salkeld SL, Popich-Patron LS, Ryaby JP, Jones DG, et al. (2001) Improved cartilage repair after treatment with low-intensity pulsed ultrasound. Clin Orthop Relat Res S231-S243.
- Jia XL, Chen WZ, Zhou K, Wang ZB (2005) Effects of low-intensity pulsed ultrasound in repairing injured articular cartilage. Chin J Traumatol 8: 175-178.
- Cook SD, Salkeld SL, Patron LP, Doughty ES, Jones DG (2008) The effect of low-intensity pulsed ultrasound on autologous osteochondral plugs in a canine model. Am J Sports Med 36: 1733-1741.
- Tien YC, Lin SD, Chen CH, Lu CC, Su SJ, et al. (2008) Effects of pulsed low-intensity ultrasound on human child chondrocytes. Ultrasound Med Biol 34: 1174-1181.
- Choi BH, Woo JI, Min BH, Park SR (2006) Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A 79: 858-864.
- Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE (2000) Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 43: 801-811.
- Lefebvre V, Peeters-Joris C, Vaes G (1990) Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta 1052: 366-378.
- Tortorella MD, Malfait AM, Deccico C, Arner E (2001) The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage 9: 539-552.
- Ito A, Aoyama T, Yamaguchi S, Zhang X, Akiyama H, et al. (2012) Low-intensity pulsed ultrasound inhibits messenger RNA expression of matrix metalloproteinase-13 induced by interleukin-1beta in chondrocytes in an intensity-dependent manner. Ultrasound Med Biol 38: 1726-1733.
- Li X, Li J, Cheng K, Lin Q, Wang D, et al. (2011) Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophys 61: 427-434.
- Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J (2007) Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol 3: 391-399.
- Zeng D, Luo Q, Lin H, Zhang J, He C (2012) The effect of therapeutic ultrasound to apoptosis of chondrocyte and caspase-3 and caspase-8 expression in rabbit surgery-induced model of knee osteoarthritis. Rheumatol Int 32: 3771-3777.
- Vincent HK, Heywood K, Connelly J, Hurley RW (2012) Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R 4: S59-S67.
- Zhuo Q, Yang W, Chen J, Wang Y (2012) Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 8: 729-737.
- Gomez R, Lago F, Gomez-Reino J, Dieguez C, Gualillo O (2009) Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. J Mol Endocrinol 43: 11-18.
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, et al. (2007) Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 102: 919-925.
- Messier SP (2008) Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am 34: 713-729.
- Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O (2008) Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol 252: 139-145.
- Christensen R, Astrup A, Bliddal H (2005) Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage 13: 20-27.
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, et al. (2004) Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 50: 1501-1510.
- Messier SP, Gutekunst DJ, Davis C, DeVita P (2005) Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum 52: 2026-2032.
- Christensen R, Bartels EM, Astrup A, Bliddal H (2007) Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 66: 433-439.
- Hotta TA (2010) Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs 30: 77-82.
- Teitelbaum SA, Burns JL, Kubota J, Matsuda H, Otto MJ, et al. (2007) Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg 120: 779-789.
- Mulholland RS, Paul MD, Chalfoun C (2011) Noninvasive body contouring with radiofrequency, ultrasound, cryolipolysis, and low-level laser therapy. Clin Plast Surg 38: 503-520.
- Haar GT, Coussios C (2007) High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 23: 89-104.