Journal of Surgery
Download PDF
Case Report
*Address for Correspondence: Vittorio Bresadola MD, Department of Surgical Sciences, General Surgery and Transplantation Unit, University Hospital of Udine, AOU S.M. della Misericordia, Piazzale Santa Maria della Misericordia,15, 33100 Udine, Italy, Tel. +39 0432 559557; Fax +39 0432 559564; E-mail: vittorio.bresadola@uniud.it
Citation: Bresadola V, Pravisani R, Terrosu G, Risaliti A. Laparoscopic Splenectomy as the Definitive Investigation for Differential Diagnosis of Elevated Serum CA 19-9 Levels Associated with a Splenic Cyst: Case Report and Review of the Literature. J Surgery. 2013;1(1): 6.
Copyright © 2013 Bresadola V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 05 August 2013 | Accepted: 27 August 2013 | Published: 30 August 2013
Reviewed & Approved by: Dr. Michael Leitman, Department of Surgery, Albert Einstein College of Medicine, USA
Laparoscopic Splenectomy as the Definitive Investigation for Differential Diagnosis of Elevated Serum CA 19-9 Levels Associated with a Splenic Cyst: Case Report and Review of the Literature
Vittorio Bresadola*, Riccardo Pravisani, Giovanni Terrosu and Andrea Risaliti
- Department of Surgical Sciences, General Surgery and Transplantation Unit, University Hospital of Udine, Udine, Italy
*Address for Correspondence: Vittorio Bresadola MD, Department of Surgical Sciences, General Surgery and Transplantation Unit, University Hospital of Udine, AOU S.M. della Misericordia, Piazzale Santa Maria della Misericordia,15, 33100 Udine, Italy, Tel. +39 0432 559557; Fax +39 0432 559564; E-mail: vittorio.bresadola@uniud.it
Citation: Bresadola V, Pravisani R, Terrosu G, Risaliti A. Laparoscopic Splenectomy as the Definitive Investigation for Differential Diagnosis of Elevated Serum CA 19-9 Levels Associated with a Splenic Cyst: Case Report and Review of the Literature. J Surgery. 2013;1(1): 6.
Copyright © 2013 Bresadola V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 05 August 2013 | Accepted: 27 August 2013 | Published: 30 August 2013
Reviewed & Approved by: Dr. Michael Leitman, Department of Surgery, Albert Einstein College of Medicine, USA
Abstract
Splenic cysts are relatively rare entities. The differential diagnosis for these lesions includes parasite infections, results of previous trauma or infarction, congenital forms, primary splenic neoplasm or cystic metastasis. They can be either symptomatic, causing mainly abdominal pain, or asymptomatic, thus being diagnosed as incidental findings during radiological examinations for other clinical reasons: among these, a raised serum level of CA 19-9 can be a case. It has been demonstrated that epidermoid and mesothelial congenital cysts can be associated with a pathological level of this tumor marker which is usually correlated to biliopancreatic and colonic carcinomas. The aim of the present study is to present the case of an asymptomatic epidermoid splenic cyst associated with a continuous increase of CA 19-9. We describe the applied clinical workup and surgical management by laparoscopic total splenectomy. Moreover, we conducted a systematic review of the literature to analyze the demographic, clinical and pathological data of these infrequent lesions and to compare our therapeutic management with that of the other reported cases.Keywords
Splenic cyst; CA 19-9; Carbohydrate antigen 19-9; Laparoscopic splenectomyIntroduction
Splenic cysts are relatively rare entities, with an incidence of 0.07% as reported in a review of 42327 autopsies [1]. The differential diagnosis for these lesions includes parasite infections, results of previous trauma or infarction, congenital forms, primary splenic neoplasm or cystic metastasis [2-11]. Moreover congenital epidermoid and mesothelial cysts show an association with increased levels of CA 19-9, a tumor marker for gastrointestinal and biliopancreatic carcinomas [12,13].In the present paper we report a case of splenic cyst associated with a continuous increase of CA 19-9 and its clinical management by laparoscopic splenectomy. A literature review of clinical characteristics and surgical treatment of splenic cysts with high level of CA 19-9 in the last 20 years is also reported.
Materials and Methods
A comprehensive review of all published cases of splenic cyst associated with elevated CA 19-9 primarily submitted to a surgical management was conducted through a systematic Medline search in the PubMed database. “Splenic cyst”, “carbohydrate antigen 19-9” and “CA 19-9” were used as key words. One case was excluded since the patient was not submitted to any surgical procedure after failure of percutaneous drainage and injection of tetracycline [14]. Furthermore we didn’t include cases of cysts arising in intrapancreatic accessory spleens [15] since they were considered not homogeneous in comparison to the present case from a diagnostic and therapeutic point of view. For all the others, demographics, clinical, surgical and pathological data were recorded and compared (Tables 1 and 2).Case Report
A 48-year-old woman in overall good clinical conditions, asymptomatic, without any comorbidity, previous abdominal trauma or recent infections, was referred to our Surgical Unit with an abnormal CA 19-9 level associated with the presence of a splenic cyst.The clinical history started with a routine gynecological checkup. Due to the clinical suspect of an ovarian neoformation, tumor markers serum dosage and an abdominal ultrasound examination were programmed. Tumor markers profile resulted as sequent (normal value): CA 19-9 318.6 U/ml (< 37), CEA 1.5 ug/L (< 5), CA 15 13.9 U/ml (< 35), CA15-3 26.3 U/ml (< 30). Abdominal ultrasound examination excluded the suspect of an ovarian lesion but revealed the presence of a splenic cyst. Serological tests for parasitic infection by Echinococcus granulosus were negative and hemocrome with formula, liver and renal function tests were normal. Physical examination was negative. Since the patient was asymptomatic, and the isolated abnormal CA 19-9 level could be a false positive, a follow up surveillance was programmed. The seriated tumor markers dosage showed a raising trend of the CA 19-9 up to 593.3 UI/ml after 4 months, therefore an MRI scan was performed. No other pathological finding could be demonstrated a part from the splenic cyst which appeared to occupy the upper and middle pole of the spleen (which was otherwise within the size range) without any relationship with the pancreatic tail, 7.5 x 8 cm in diameter, not associated with any solid mass and filled of a proteinous content (Figure 1). No percutaneous fine needle aspiration was planned. In accordance with the oncologists we decided to perform a laparoscopic splenectomy to make sure the relationship between the marker’s level and the cyst and to exclude any potential malignant origin (either occult primary or secondary). No intraoperative complication occurred. The postoperative course was uneventful and the patient was discharged on postoperative day 3. The level of CA 19-9 in the cystic content was 6938970 U/ml. Histological examination confirmed the diagnosis of a benign epidermoid cyst. In the immunostaining analysis the epithelium was positive for CA 19-9 and CEA. During the follow up the CA 19-9 value progressively decreased and became normal 2 months after splenectomy, conclusively excluding any other concomitant cause for its initial abnormal level. Perioperative trend of the tumor marker is described in Figure 2.
Discussion
Carbohydrate antigen 19-9 (CA 19-9) is a glycoprotein produced in ductal epithelial cells of salivary glands, biliary and pancreatic ducts and in metaplastic mesothelial cells. Elevated levels are associated with pancreatic, biliary and gastrointestinal carcinomas [10,11,16]. In these cases CA 19-9 is used for diagnosis and as surveillance marker in the follow up with a sensitivity and specificity of 77-88% and 84-90% respectively [3,17-24]. However also benign conditions such as cirrhosis, cholangitis, cystic fibrosis, pancreatitis can determine abnormal CA 19-9 levels, resulting in false positive data [18,19,25,26]. Combining the mechanism of pathogenesis and the microscopic characteristics, splenic cysts are classified as parasitic (maily caused by Echinococcus granulosus infection) or non-parasitic. The latter are further subdivided into primary or secondary in relation to the presence or absence of an epithelial lining. Secondary cysts or pseudocysts are usually post-traumatic or caused by infarction of the spleen. Primary non-parasitic cysts can be congenital or neoplastic. The histological features of the epithelial lining within congenital forms imply an additional subclassification:- epidermoid cyst (stratified non keratinizing squamous epithelium), which accounts for 90% of congenital cyst
- dermoid cyts (squamous lining with dermal structures)
- mesothelial cysts (low cuboidal or low columnar epithelium)
- angiomatous cyst (derived from endothelium) which represents lymphangiomas and hemangioma [2-11]
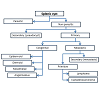
Primary non-parasitic splenic cyst can also be of neoplastic nature, either primary or secondary [6,10,27-29]. There have been reported in literature cases of primary splenic cystadenocarcinomas [27,28] and of splenic lymphoma presenting as a splenic cyst [30,31]. Isolated splenic metastasis are diagnosed in only 5.2% of case on autoptic studies [32,33]. They are mostly secondary to melanoma and cancer of the breast, lung, ovary, colon, stomach and pancreas [32-35]. Although they usually appear as solid lesions, hemorrhagic phenomena, cystic or necrotic degenerations can occur, conferring to the metastasis a cystic feature [34-37]. Moreover a cystic adenomaadenocarcinoma of the pancreatic tail, extended within the splenic parenchyma should be excluded [10,38]. A simplified diagram of the classification of splenic cysts is reported in Figure 3.
The first report about the association between serum levels of CA 19-9 and a splenic cyst was in 1994: a case of a young woman with a huge splenic cyst and a marker level of 800 U/ml who was submitted to open splenectomy with subsequent CA 19-9 level normalization [12]. Since then, similar clinical pictures have been reported in a small number of cases (Table 1). The pathogenesis responsible for this correlation could be explained by the demonstration with immunohistochemical analyses that the epithelium lining primary epidermoid cyst produces and secretes CA 19-9 or other tumor markers [3,5,6,8-11,18,29,39]. The increased level would be the result of diffusion phenomena of the antigens from the liquid content of the cyst to the vascular system. Hence the serum concentration would depend on the degree of proliferation of the epithelium, capsule thickness, and presence of capillaries in connective tissue surrounding the cyst [3,8,10,18,29].
It has been reported in several cases that also mesothelial cyst can be associated to elevated CA 19.9 serum level [3,13,40].
Because of the limited number of cases reported in literature, it was not possible to evaluate with a statistical significance the possible correlation between serum level of CA 19-9, intracystic level and cystic dimension. However by descriptive analysis it seems that these parameters are not reciprocally dependent. As reported, other tumor markers, particularly CA 125 and CEA, are associated with epidermoid/mesothelial cysts (Table 1).
This is the first case report where the trend of CA 19-9 levels is preoperatively monitored over time. This additional data with its increasing trend was a relevant element in the clinical management of the case, considering the asymptomatic state of the patient.
The low prevalence of splenic cysts and the incompletely understood pathogenesis of the CA 19-9 secreting epithelium must be contrasted to the well-established association between CA 19-9 and biliopancreatic carcinoma [3,19,22] (despite negative imaging investigation). Thus the possibility to definitively exclude a concomitant life-threatening disease through cyst resection or splenectomy may, in our opinion, represent a crucial indication for surgical intervention. Moreover it is particularly relevant in the case of young patients as those epidemiologically most affected by splenic cysts (Table 1).
In our case, percutaneous aspiration of the cyst was refused either as diagnostic or therapeutic tool since this procedure not only doesn’t offer any conclusive diagnostic information but also carries the risk of recurrence, rupture, abscess formation [8,14,22,41-43] and of seeding malignant cells into the peritoneum or along the needle tract [6].The review of the demographics data shows that splenic cysts with raised CA-19.9 level, when symptomatic, cause mainly upper abdominal discomfort/pain, either acute or chronic. The present case and other two [13,42] are the only reports with asymptomatic patients. Female young adults are the most frequent patients: mean age is 25 years old (18-46) and female sex represents the 85.2% of cases. At diagnosis, mean CA-19.9 serum level is as high as 6770.9 UI/ml (65-85000) which should be compared to the record that a serum level higher then 1000 U/ml has a specificity of 99% for pancreatic cancer [3]. Maximum diameter at radiological examination is 16.3 cm (3-28). Acute complications are represented by rupture, hemorrhage, abscessualization and constitute the clinical picture at diagnosis in the 11.1% of cases (Table 1).General indications for surgery are the presence of symptoms, acute complications or increased risk of complications (diameter > 5 cm) [5,6,8,10,18,22,43]. Timing and types of treatment for asymptomatic splenic cysts remain unclear in the absence of standard guidelines [8,10,22].
In general, the proposed surgical modalities for treatment include splenectomy, partial splenectomy, marsupialisation, cystectomy and cystic decapitation [6,18,26,29,42,43]. The rationale sustaining spleen preserving procedures is the possibility to minimize the infectious risks associated with the loss of splenic function [6,26,29,44]. However the routine use of vaccination against capsulated bacteria post splenectomy has reduced such risk. Furthermore, if it is taken into consideration that the mean dimension at diagnosis of the epidermoid cysts is 16.3 cm (Table 1), the possibility to leave a viable and functioning splenic remnant (>25% in volume) appears difficult to perform as the majority of the spleen has already been substituted by the cyst [10,11,22,26,29,42-46]. As a matter of fact, even a minimally invasive approach was used just in 39% of cases (if included also our case) probably due to clinical complications such as rupture or technical difficulties secondary to the cyst’s dimension or adhesion. On the other hand spleen-preserving procedures are actually reported to be associated with several and critical risks: intraoperative and postoperative hemorrhage to due insufficient hemostasis, recurrence and incomplete oncological radicality [4,6,9,11,29,40,43,46,47]. Conclusively, although a spleen preserving approach would be desirable, a total splenectomy was actually performed in 81.5% cases (Table 2) which practically confirms, in agreement with our management, that the associated morbidity of these procedures represents at the moment a major limit for their application.
These are the reasons why we currently consider laparoscopic splenectomy the gold standard for the treatment of splenic cysts associated with increased CA 19-9. In particular, laparoscopy for splenic surgery has been established as a safe and feasible approach which can reduce morbidity and mortality, shorter hospital stay and hasten functional recovery [4-6,11,26,43,48].
After surgical resection, all cases demonstrated a decreasing trend of the CA-19.9 serum level, ascertaining a definitive etiologic correlation with the resected splenic cyst. Average interval between operation and normalization of tumor marker’s dosage was 9.2 weeks (Table 2). Although the association between elevated CA 19.9 serum levels and splenic cysts proved to be a benign condition in all the reported cases, this diagnosis could be established just retrospectively after surgical radical resection and normalization of the tumor maker level, thus confirming again splenectomy as the definitive investigation for a conclusive differential diagnosis.
References
- Robbins FG, Yellin AE, Lingua RW, Craig JR, Turrill FL, et al. (1978) Splenic epidermoid cysts. Ann Surg 187: 231-235.
- van Lacum MW, Hessels RA, Kremer GD, Jaspers CA (2000) A splenic cyst and a high serum CA 19-9: a case report. Eur J Intern Med 11: 104-107.
- Trompetas V, Panagopoulos E, Priovolou-Papaevangelou M, Ramantanis G (2002) Giant benign true cyst of the spleen with high serum level of CA 19-9. Eur J GastroenterolHepatol 14: 85-88.
- Tagaya N, Oda N, Furihata M, Nemoto T, Suzuki N, et al. (2002) Experience with laparoscopic management of solitary symptomatic splenic cysts. Surg Laparosc Endosc Percutan Tech 12: 279-282.
- Madia C, Lumachi F, Veroux M, Fiamingo P, Gringeri E, et al. (2003) Giant splenic epithelial cyst with elevated serum markers CEA and CA 19-9 levels: an incidental association? Anticancer Res 23: 773-776.
- Hansen MB, Moller AC (2004) Splenic cysts. Surg Laparosc Endosc Percutan Tech 14: 316-322.
- Mirilas P, Mentessidou A, Skandalakis JE (2007) Splenic cysts: are there so many types? J Am Coll Surg 204: 459-465.
- Inokuma T, Minami S, Suga K, Kusano Y, Chiba K, et al. (2010) Spontaneously Ruptured Giant Splenic Cyst with Elevated Serum Levels of CA 19-9, CA 125 and Carcinoembryonic Antigen. Case Rep Gastroenterol 4: 191-197.
- Papadopoulos IN, Davatzikos A, Kasabalis G, Manti C, Konstantoudakis G (2010) Primary epithelial splenic cyst with micro-rupture and raised carbohydrate antigen CA 19-9: a paradigm of management. BMJ Case Rep.
- Brauner E, Person B, Ben-Ishay O, Kluger Y (2012) Huge splenic cyst with high level of CA 19-9: the rule or the exception? Isr Med Assoc J 14: 710-711.
- Vo QD, Monnard E, Hoogewoud HM (2013) Epidermoid cyst of the spleen. BMJ Case Rep.
- Terada T, Yasoshima M, Yoshimitsu Y, Nakanuma Y (1994) Carbohydrate antigen 19-9 producing giant epithelial cyst of the spleen in a young woman. J Clin Gastroenterol 18: 57-61.
- Walz MK, Metz KA, Sastry M, Eigler FW, Leder LD (1994) Benign mesothelial splenic cyst may cause high serum concentration of CA 19-9. Eur J Surg 160: 389-391.
- Yoshikane H, Suzuki T, Yoshioka N, Ogawa Y, Hayashi Y, et al. (1996) Giant splenic cyst with high serum concentration of CA 19-9. Failure of treatment with percutaneous transcatheter drainage and injection of tetracycline. Scand J Gastroenterol 31: 524-526.
- Fujishiro J, Kaneko M, Urita Y, Hoshino N, Jinbo T et al. (2011) Enteric duplication cyst of the pancreas with duplicated pancreatic duct. J Pediatr Surg 46: e13-e16.
- Urban D, Catane R (2009) Serum tumor markers in oncology. Isr Med Assoc J 11: 103-104.
- Ishibashi R, Sakai T, Yamashita Y, Maekawa T, Hideshima T, et al. (1999) Benign epithelial cyst of the spleen with a high production of carbohydrate antigen 19-9. Int Surg 84: 151-154.
- Lieto E, Castellano P, Ferraraccio F, Orditura M, De Vita F, et al. (2003) Normal interleukin-10 serum level opposed to high serum levels of carbohydrate antigen 19-9 and cancer antigens 125 and 50 in a case of true splenic cyst. Arch Med Res 34: 145-148.
- Soudack M, Ben-Nun A, Toledano C (2001) Elevated carbohydrate antigen 19-9 in patients with true (epithelial) splenic cysts--Rare or undiscovered? Can J Gastroenterol 15: 125-126.
- Qin XL, Wang ZR, Shi JS, Lu M, Wang L, et al. (2004) Utility of serum CA 19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol 10: 427-432.
- Goonetilleke KS, Siriwardena AK (2007) Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J SurgOncol 33: 266-270.
- Uludag M, Yetkin G, Citgez B, Karakoc S, Polat N, et al. (2009) Giant true cyst of the spleen with elevated serum markers, carbohydrate antigen 19-9 and cancer antigen 125. BMJ Case Rep.
- Molina V, Visa L, Conill C, Navarro S, Escudero JM, et al. (2012) CA 19-9 in pancreatic cancer: retrospective evaluation of patients with suspicion of pancreatic cancer. Tumour Biol 33: 799-807.
- Mourtzikou A, Stamouli M, Kroupis C, Christodoulou S, Skondra M, et al. (2012) Evaluation of carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9) levels in colorectal cancer patients and correlation with clinicopathological characteristics. Clin Lab 58: 441-448.
- Ito S, Gejyo F (1999) Elevation of serum CA 19-9 levels in benign diseases. Intern Med 38: 840-841.
- Paksoy M, Karabicak I, Kusaslan R, Demiryas S, Ayan F, et al. (2006) Laparoscopic splenic total cystectomy in a patient with elevated CA 19-9. JSLS 10: 507-510.
- Elit L, Aylward B (1989) Splenic cyst carcinoma presenting in pregnancy. Am J Hematol 32: 57-60.
- Morinaga S, Ohyama R, Koizumi J (1992) Low-grade mucinous cystadenocarcinoma in the spleen. Am J Surg Pathol 16: 903-908.
- Palmieri I, Natale E, Crafa F, Cavallaro A, Mingazzini PL (2005) Epithelial splenic cysts. Anticancer Res 25: 515-521.
- Nakashima A, Nakashima K, Seto H, Kamei T, Kakishita M, et al. (1994) Primary splenic lymphoma presenting as a large cyst. Radiat Med 12: 42-45.
- Takabe K, Al-Refaie W, Chin B, Chu PK, Baird SM, et al. (2005) Can large B-cell lymphoma mimic cystic lesions of the spleen? Int J Gastrointest Cancer 35: 83-88.
- Lam KY, Tang V (2000) Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med 124: 526-530.
- Schon CA, Görg C, Ramaswamy A, Barth PJ (2006) Splenic metastases in a large unselected autopsy series. Pathol Res Pract 202: 351-356.
- Comperat E, Bardier-Dupas A, Camparo P, Capron F, Charlotte F (2007) Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med 131: 965-969.
- Delaunoit T, Peny MO, Mignon M, Dili A (2012) Splenic metastasis from gastrointestinal neoplasms: a review. Acta Gastroenterol Belg 75: 3-4.
- Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR (1996) Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics 16: 107-129.
- Kaza RK, Azar S, Al-Hawary MM, Francis IR (2010) Primary and secondary neoplasms of the spleen. Cancer Imaging 10: 173-182.
- Adas G, Karatepe O, Altiok M, Battal M, Bender O, et al. (2009) Diagnostic problems with parasitic and non-parasitic splenic cysts. BMC Surg 9: 9.
- Sakamoto Y, Yunotani S, Edakuni G, Mori M, Iyama A, et al. (1999) Laparoscopic splenectomy for a giant splenic epidermoid cyst: report of a case. Surg Today 29: 1268-1272.
- Sardi A, Ojeda HF, King D Jr. (1998) Laparoscopic resection of a benign true cyst of the spleen with the harmonic scalpel producing high levels of CA 19-9 and carcinoembryonic antigen. Am Surg 64: 1149-1154.
- Mahomed A, Merry C, Guiney EJ (1998) Splenic cysts--aspiration or partial splenic decapsulation? S Afr J Surg 36: 84-86.
- Morandi E, Castoldi M, Merlini DA, Vignati G, Milanesi M (2012) Is there a role of percutaneous drainage in non-parasitics plenic cysts? Case report. G Chir 33: 343-345.
- Yoh T, Wada S, Kobayashi A, Nakamura Y, Kato T, et al. (2013) Laparoscopic splenectomy for a large multilocular splenic cyst with elevated CA 19-9: Report of a case. Int J Surg Case Rep 4: 319-321.
- Kaiwa Y, Kurokawa Y, Namiki K, Matsumoto H, Satomi S (2000) Laparoscopic partial splenectomies for true splenic cysts. A report of two cases. Surg Endosc 14: 865.
- Di Carlo I, Fasone MA, Toro A (2005) Epidermoid cyst of the spleen in the laparoscopic era. Dig Surg 22: 53-54.
- Galizia G, Lieto E, Ferraraccio F, Castellano P, De Vita F, et al. (2003) A true splenic cyst producing carbohydrate antigen 19-9 and cancer antigens 50 and 125, but not interleukin 10. Dig Surg 20: 71-74.
- Balzan SM, Riedner CE, Santos LM, Pazzinatto MC, Fontes PR (2001) Posttraumatic splenic cysts and partial splenectomy: report of a case. Surg Today 31: 262-265.
- Chiarugi M, Galatioto C, Battini A, Panicucci S, Lippolis P, et al. (2006) Giant epidermoid cyst of the spleen with carbohydrate and cancer antigen production managed laparoscopically. Ann Ital Chir 77: 443-446.
- Higaki K, Jimi A, Watanabe J, Kusaba A, Kojiro M (1998) Epidermoid cyst of the spleen with CA 19-9 or carcinoembryonic antigen productions: report of three cases. Am J Surg Pathol 22: 704-708.
- Hashimoto T, Sugino T, Fukuda T, Hoshi N, Ogura G, et al. (2004) Multiple epithelial cysts of the spleen and on the splenic capsule, and high serum levels of CA 19-9, CA125 and soluble IL-2 receptor. Pathol Int 54: 349-354.
- Matsubayashi H, Kuraoka K, Kobayashi Y, Yokota T, Iiri Y, et al. (2001) Ruptured epidermoid cyst and haematoma of spleen: a diagnostic clue of high levels of serum carcinoembryonic antigen, carbohydrate antigen 19-9 and Sialyl Lewis x. Dig Liver Dis 33: 595-599.
- Yigitbasi R, Karabicak I, Aydogan F, Erturk S, Bican O, et al. (2006) Benign splenic epithelial cyst accompanied by elevated Ca 19-9 level: a case report. Mt Sinai J Med 73: 871-873.