Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
*Address for Correspondence: Tamara Minko, Distinguished Professor and Chair, Department of Pharmaceutics, Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey 160 Frelinghuysen Road Piscataway, NJ 08854–8020, USA, Tel: 848-445-6348; Fax: 732-445-3134; E-mail: minko@rci.rutgers.edu
Citation: Shah M, Shah V, Ghosh A, Zhang Z, Minko T. Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility andCellular Internalization of Paclitaxel: Preformulation and In vitro Assessments. J Pharmaceu Pharmacol. 2014;2(2): 8.
Copyright © 2014 Shah M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology |ISSN: 2327-204X | Volume: 2, Issue:2
Submission: 27 November 2014| Accepted: 05 January 2015 | Published: 10 January 2015
Reviewed & Approved by: Dr. Rutesh H. Dave, Associate Professor of Pharmaceutical Sciences, Arnold and Marie Schwartz College of Pharmacy and Health sciences, Long Island University, USA
Keywords: Solubility; Enhancement; Inclusion; Compounds; Permeability enhancement; Cyclodextrins; Preformulation
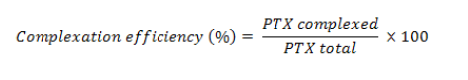
Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility and Cellular Internalization of Paclitaxel: Preformulation and In vitro Assessments
Milin Shah1, Vatsal Shah1, Anasuya Ghosh1, Zheng Zhang2,3, Tamara Minko1*
- 1Department of Pharmaceutics, Rutgers, The State University of New Jersey, Piscataway, NJ, USA2Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, Piscataway, NJ, USA33New Jersey Center for Biomaterials, Rutgers, The State University of New Jersey, Piscataway, NJ, USA
*Address for Correspondence: Tamara Minko, Distinguished Professor and Chair, Department of Pharmaceutics, Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey 160 Frelinghuysen Road Piscataway, NJ 08854–8020, USA, Tel: 848-445-6348; Fax: 732-445-3134; E-mail: minko@rci.rutgers.edu
Citation: Shah M, Shah V, Ghosh A, Zhang Z, Minko T. Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility andCellular Internalization of Paclitaxel: Preformulation and In vitro Assessments. J Pharmaceu Pharmacol. 2014;2(2): 8.
Copyright © 2014 Shah M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology |ISSN: 2327-204X | Volume: 2, Issue:2
Submission: 27 November 2014| Accepted: 05 January 2015 | Published: 10 January 2015
Reviewed & Approved by: Dr. Rutesh H. Dave, Associate Professor of Pharmaceutical Sciences, Arnold and Marie Schwartz College of Pharmacy and Health sciences, Long Island University, USA
Abstract
Drugs with low aqueous solubility and permeability possess substantial challenges in designing effective and safe formulations. Synergistic solubility and permeability enhancement in a simple formulation can increase bioavailability and efficacy of such drugs. To overcome limitations of the clinical formulation of Taxol®, Paclitaxel (PTX) was reformulated with various β-cyclodextrin(CD) derivatives suitable for parenteral administration.Results indicated that β-CDs can efficiently form complexes with PTX at lower molar ratios, enhance aqueous solubility up to 500 times and improved cellular internalization of PTX. All β-CD derivatives were found to be safe as excipient since none showed detectable signs of cyto-genotoxicity. As a result, the CD-PTX complexes significantly increased the cytotoxicity of the drug. The study concluded that CD-PTX formulations could substitute the current intravenous infusion of PTX obviating the use of non-inert excipient Cremophor EL.Keywords: Solubility; Enhancement; Inclusion; Compounds; Permeability enhancement; Cyclodextrins; Preformulation
Introduction
A mitotic inhibitor, Paclitaxel (PTX), is a potent, natural diterpenoid anticancer drug with unique mechanism of action. Unfortunately, PTX has very low aqueous solubility and belongs to the class IV in the biopharmaceutical classification system (BCS) [1]. According to BCS, drugs belonging to class IV are the most difficult to formulate into a successful clinical formulation owing to low solubility and permeability [2]. PTX was approved by the US Food and Drug Administration in 1992 under the brand name Taxol® indicated for ovarian and breast carcinoma, non-small cell lung cancer and AIDS related Kaposi’s sarcoma. Taxol® is a nonaqueous solution that uses a mixture of Cremophor EL and Ethanol (1:1) as a vehicle to solubilize PTX. According to the label insert, the formulation should be diluted in normal saline or 5% (w/v) dextrose solution for intravenous administration by slow infusion over 3 to 24 hours. Severe hypersensitivity reactions, neutropenia, nephrotoxicity and neurotoxicity are some of the major side effects of Cremophor EL [3]. Consequently, to avoid the risk of such side effects, a prophylactic treatment with antihistamines and corticosteroids is necessary prior to Taxol® infusion [4]. The prophylactic medication may reduce the severity of the hypersensitivity reaction, but cannot completely eliminate it [4,5]. The infusion mixture was reported to have stability issues and Cremophor EL has been found responsible for leaching of plasticizers from polyvinyl chloride infusion set [6]. Hence, the development of safe formulation of PTX represents an important goal in anticancer drug improvement and there is a crucial need to reformulate poorly water-soluble PTX in a simple formulation. Reformulation is a suitable and cost effective approach to avoid the use of Cremophor EL and thereby associated side effects, stability issues, and possibly a prophylactic regimen administered prior to Taxol® treatment.β-cyclodextrin (CD) is a cyclic heptamer of glucose α-1,4 D-glucopyranoside that creates a toroid structure with a hollow hydrophobic core. Macrocyclic hosts such as CD are used to prepare supramolecular delivery systems by forming non-covalent inclusion complexes with a variety of drug molecules [7,9]. These inclusion complexes exhibit higher aqueous solubility and thermodynamic stability for the complexed drug molecule [10]. Several derivatives of CD are reported as non-immunogenic, biocompatible, and suitable for human use by various regulatory agencies including the United States Food and Drug Administration (USFDA) [11]. Studies have demonstrated that PTX complexed with CD not only showed higher aqueous solubility but also had better stability [12]. Therefore, CD can potentially provide a suitable alternative to Cremophor EL-based formulations for PTX to substitute the current formulations in the clinical settings.
The objective of the study was to overcome the limitations of Cremophor EL-based formulations with molecular inclusion complexes CD-PTX suitable for parenteral administration. Such CD-based formulations were investigated to determine the solubility enhancement and study solid-state physicochemical interactions. Furthermore, CD-PTX complexes were tested in vitro to analyze their cytotoxicity by modified MTT (3-(4,5 dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) assay. Fluorescent microscopy and flow cytometric studies were performed to assess PTX cellular internalization. Finally, safety of the drug-free CDs as excipients was determined by qualitative and quantitative cyto- and genotoxicity study.
Materials and Methods
MaterialsPaclitaxel was purchased from Sigma Aldrich (St. Louis, MO). Hydroxypropyl β-cyclodextrin (HPCD, Kleptose®) and Methyl β-cyclodextrin (MeCD, Crysmeb) were purchased from Roquette Pharma (Lestrem, France). Sulfobutyl ether β-cyclodextrin (SBCD) was purchased from Cydex Pharmaceuticals (Lenexa, KS). Tertbutyl alcohol was purchased from Alfa Aesar (Ward Hill, MA). Cyclophosphamide (CP), Ethylmethyl sulfone (EMS), Glucose 6-phosphate, Acetonitrile, Methanol Dimethyl Sulfoxide (DMSO), Tween 20, 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI), Thiazolyl blue tetrazolium bromide, (MTT), Sodium dodecyl sulfate(SDS) and Dimethyl formamide (DMF) were purchased from Sigma Aldrich (St. Louis, MO). Nicotinamide adenine dinucleotide phosphate (NADP) was obtained from Fisher Chemicals (Suwannee, GA).
Cell culture
PC-3 human prostate adenocarcinoma, A549 human lung adenocarcinoma, HT-29 human colorectal adenocarcinoma, MCF- 7 human breast adenocarcinoma and Chinese hamster ovary-K1 (CHO-K1) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). A2780 human ovarian carcinoma cell line was obtained from Dr. T.C. Hamilton (Fox Chase Cancer Center). CHO-K1 cells were cultured in F-12K medium (ATCC, Manassas, VA) with 10% Fetal bovine serum (Life Technologies, Grand Island, NY) and penicillin-streptomycin solution (100 UI/mL- 100 μg/mL, Life Technologies). All other cells were cultured in RPMI- 1640 medium (Life Technologies) with 10% Fetal bovine serum and penicillin-streptomycin solution (100 UI/mL-100 μg/mL). All cells were grown in humidified conditions with 5% CO2 (v/v) in air. The cell-based experiments were performed on cells in the exponential growth phase..
Preparation of inclusion complexes
The inclusion complexes of PTX and CD were prepared using a modified co-solvent lyophilization method [12]. Three molar ratios of PTX and CD were chosen for the experiment as 1:1, 1:2 and 1:5, respectively. Briefly, in one vial required quantity of CD was dissolved in 5 mL deionized water. In another vial, 8.5 mg of PTX was added followed by 100 μL of Acetonitrile and 400 μL of Tert butyl alcohol. Both phases were vortexed thoroughly to ensure clear solutions followed by addition of PTX solution to the CD solution in a drop-wise manner while stirring. The resultant mixture was kept for six hours at room temperature under stirring. With regards to the entrapment efficiency, six hours of stirring was found to be optimal. Stirring duration of 3 hours or less resulted in low entrapment efficiency. Hence, further experiments were performed using optimized duration of six hours of stirring. After six hours, the mixture was filtered using 0.2 μm syringe filter and lyophilized overnight. The resultant lyophilized formulation was stored in glass vials at 2-8 °C until further use.
Entrapment efficiency and solubility enhancement
To determine PTX entrapment in the inclusion complexes, a modified chromatographic method was used [13]. The chromatographic equipment consisted of an autosampler (Waters 717 plus Autosampler), a pump (Waters 1525 binary pump) and a detector (Waters 2487 dual λ detector). The samples were run using a mobile phase of water:methanol (35:65) at a flow rate of 1 mL/min with detection wavelength of 227 nm using C18 column (3.9 mm x 150 mm, Waters) at 25 °C. The lyophilized formulations were dissolved in water and centrifuged at 10,000 rpm for 5 min. The supernatant was collected and subjected to measurements. Entrapment efficiency was calculated using the following formula
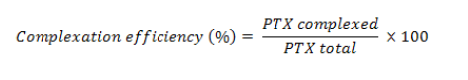
Standards of Paclitaxel were prepared by dissolving Paclitaxel in 95% Ethanol. PTX calibration curve was made ranging from 1-50 μg/mL. Solubility of pure PTX in aqueous suspension was also determined using the above HPLC method. The aqueous solubility of PTX was determined to be 0.38 μg/mL at 25 °C.
Physicochemical evaluation
Differential scanning calorimetry (DSC) was used to evaluate drug-excipient interactions and confirm complexation. The studies were performed using a differential scanning calorimeter (Mettler Toledo) at the heating rate of 2 °C/min for each sample. PTX, three CD samples and physical mixtures of CD and PTX were studied as controls. Powdered X-ray diffraction studies (XRD) were performed to evaluate the crystal structure of the drug within the complexes using Bruker D8 Advance x-ray diffractometer (Bruker AXS Inc., Madison, WI). 1HNMR studies were performed on complexes using Bruker 700 MHz advanced spectrometer (Bruker Instruments, Billerica, MA) with PTX as a control.
Genotoxicity
Modified in vitro micronuclei test was used to evaluate genotoxic potential of cyclodextrins. The test was adopted from Organization for Economic Cooperation and Development (OECD) protocol and performed as previously described [14]. Briefly, in a 25 cm2 flask, about 300,000 CHO-K1 cells were cultured for 24 hours before treatment. After 24 hours, the cells were treated with CDs (50 mg/ mL) for three hours followed by replacement of fresh media and incubation for another 24 h. Then, the cells were stained to detect the presence of micronuclei. The set of experiments included following series of samples: (1) Negative control – media only; (2) DMSO (100 μL) –negative control for the solvent; (3) Cyclophosphamide (Cyc) 10 mg/mL with metabolic activator (S9 mix, 0.3 mL) – positive control; (4) Ethyl methanesulfone (EMS) 400 μg/mL – positive control that does not require a metabolic activation; (5) CD samples 50 mg/mL and (6) CD (50 mg/mL) with S9 mix (0.3 mL). The S9 mixture is a metabolic activation system that enables the detection of mutagenic activity for the samples that require metabolic transformation (e.g. cyclophosphamide). In order to prepare the S9 mix, following chemicals were added to a rat liver S9 fraction (0.2 mL) after Arclor-1254 induction (purchased from Moltox, Boone, NC): sterile water for injection (560 μL), sodium phosphate buffer (0.1 M, pH 7.4, 1 mL), 4mM NADP (150 μL), glucose-6-phosphate (120 mM, 14 μL) and potassium-magnesium salt solution (8 mM – 33 mM, 60 μL). After incubation, the cells were fixed in a cold solution of 100% methanol. Methanol was removed, cells were washed with phosphate buffer, and nuclei staining was performed using 600 nM 4,6 diamidino-2- phenylindole (DAPI) for 8 minutes. After 8 minutes the solution was removed and cells were washed using PBS with 0.02% v/v Tween 20. The micronuclei were observed under fluorescent microscope and the number of micronuclei observed per 1000 cells was calculated.
Cytotoxicity
Cytotoxicity studies of PTX and inclusion complexes were performed using modified MTT (3-(4,5 dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay as previously described [15]. All cell lines (PC-3, A2780, MCF-7, HT-29 and A549) were subjected to similar treatment for the MTT assay. For the assay, 10,000 cells were seeded per well in 96-well plates and incubated for 24 hours. Next day, the media was replaced with fresh media; test substances were added and incubated for 24 hours. After 24 hours, the solution was again replaced by fresh media and MTT reagent (25 μL of 5 mg/mL). Plates were further incubated in cell culture conditions for 3 hours and the formazan crystals were dissolved overnight by adding 50% (v/v) dimethylformamide in water with 20% (w/v) sodium dodecyl sulfate. The absorbance was measured at 570 nm with background correction at 630 nm.
Cellular internalization
To estimate the cellular internalization, CD complexes were prepared (molar ratio 1:5) with Paclitaxel-Oregon Green® 488 conjugate (Flutax-2, Molecular Probes®, Life Technologies, Carlsbad, CA). For cell internalization studies, 10,000 cells (PC-3) were seeded per well in a six well plate and incubated overnight. Next day, the media was replaced and inclusion complexes were added to wells (equivalent to 1 μM of Flutax-2). Flutax-2 alone was used as a control. For the flow cytometry studies, the cells (PC-3) were cultured in a reduced serum media at 500,000 cells per well in a six well plate. Next day, the cells were washed with DPBS and fresh media with inclusion complexes (equivalent to 10 μM Flutax-2) was added. The cells were incubated for 3 hours. After incubation, the cells were harvested and washed using DPBS and re-suspended in 1 mL of reduced serum media for fluorescence assisted cell sorting (FACS) analysis using Gallios flow cytometer (Beckman Coulter, Brea, CA). Cells treated with Flutax-2 alone were used as a control.
Statistical analysis
Data were analyzed using descriptive statistics and singlefactor ANOVA, and are presented as a mean ± SD from five to ten independent measurements. We analyzed data sets for significance with Student’s t test and considered P values of less than 0.05 as statistically significant.
Cellular internalization
The main objectives of the solid-state physicochemical study were to: (a) confirm CD-PTX complexation and (b) study chemical interactions that may affect the stability and/or activity of complexed PTX. When the physical mixtures of PTX and CD were subjected to DSC studies, a sharp PTX melting point peak was observed which was absent in all three complexes. Thus, the absence of PTX melting point peak complexes confirmed PTX complexation and possibly amorphous state of the drug. In complexes, the thermograms did not show any signs interactions between CD and PTX. The amorphous nature of PTX could be attributed to CDs, where CDs prevented formation of crystalline PTX due to complexation. The powdered XRD patterns of the complexes provided additional data confirming that PTX was indeed in amorphous form in the complexes. In the 1HNMR studies, characteristic resonance peaks of PTX in the region of 7.5-8.5 ppm also indicated that PTX was effectively complexed within the CD cavity. Together DSC, 1HNMR and XRD studies have demonstrated that: (a) PTX was efficiently complexed with the CDs, (b) the complex was mainly formed due to weak hydrophobic interactions between the CDs and PTX and (c) PTX is in amorphous form within the complex. The XRD of SBCD complexes showed faint signals, which suggested that there may be a small quantity of PTX in a crystalline form possibly due to high degree of substitution on SBCD.
The cytotoxicity studies of free CDs showed that CDs lack any cytotoxic potential and can be considered safe for such delivery systems unlike Cremophor EL [19]. The cytotoxicity data also confirmed thatPTX activity remained unchanged after complexation. Lower IC50 values (higher cytotoxicity) of complexed PTX can be explained by enhanced solubility of PTX which makes more soluble drug available at the cell surface for internalization. However, the enhancement of cytotoxicity of PTX cannot be attributed solely to the enhancement in solubility of the complexes because the drug belongs to BCS class IV. As PTX exhibiting poor solubility as well as permeability, some other factors might play a role in facilitating its permeation through cell membrane once it is solubilized. To understand the effect of CDs on cellular internalization, fluorescent microscopy and flow cytometry studies were carried out. Both the studies showed that higher cellular internalization of PTX was achieved in the presence of CDs. As a possible explanation of the increased permeability of cellular membranes for the CD-PTX complexes, one can hypothesize that CDs could interact with cell membrane cholesterol and alter membrane fluidity to create transient channels for internalization of PTX across the membrane. In the vicinity of the cell membrane,such complexes interact with membrane cholesterol and release a molecule of PTX simultaneously. The released molecule of PTX will have to face less resistance from the membrane and will be easily internalized. In turn, the higher accumulation of PTX inside the cellsenhanced cytotoxicity of the complexes. The above results indicating interactions of CD and cell membrane cholesterol extended the study to assess the safety of CDs as excipients. The genotoxicity studies of CDs were performed using the micronuclei test as per the OECD guidelines. OECD has two approaches described under the genotoxicity study: (a) Structural aberration study (Test # 473,In vitro mammalian cell chromosomal aberration test) and (b) Micronuclei assay (Test # 487, In vitro mammalian cell micronucleus test). The micronucleus test was chosen to evaluate CDs since it is a better indicator of genotoxicity potential as well as the test is rapid, easy, and allows quantification of data to arrive at discrete conclusion. The aberration assay is more qualitative as it needs observational assessment of nucleus morphology to differentiate an arrested cell in the process of cell division. The number of micronuclei formation in the genotoxicity studies concluded that none of CDs found to be genotoxic. Thus, the CDs used in the present investigation were found to be safe and showed no cytotoxic or genotoxic effects.
Results
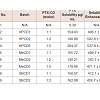
Solubility enhancement and entrapment efficiencyAll lyophilized PTX complexes were reconstituted into clear aqueous solutions of 0.2 mg/mL concentration of PTX at 25 °C. In all 9 batches, CD derivatives were found to significantly increase aqueous solubility of Paclitaxel up to 500 folds as compared to the free drug [table 1]. A general trend was observed that as the concentration of CD was increased, the solubility of PTX improved as well. In all the batches prepared, PTX showed high degree of entrapment efficiency, especially in HPCD and SBCD based complexes where more than 90% of PTX were complexed. While in MeCD, more than 80% of the PTX was entrapped in complexes.
Physicochemical evaluations of inclusion complexes
All of the physicochemical interaction studies were performed on inclusion complexes of the highest molar ratio studied of CD to PTX (5:1). In the DSC studies, thermograms of lyophilized complexes were compared with free PTX, CD alone and physical mixtures of PTX with different CDs [Figure 1]. A sharp endothermic melting peak for PTX was evidently absent in the complexes while thermograms of the physical mixtures showed the PTX melting point peak between 220 and 230 °C. In 1HNMR studies, the inclusion complexes showed small characteristics resonance of PTX due to aromatic hydrogen atoms between 7.5 and 8.5 ppm [Figure 2]. The XRD data displayed no characteristic crystalline nature of PTX in any of the complexes as compared to free PTX [Figure 3].
Genotoxicity of cyclodextrins
In vitro formation of micronuclei, a DNA fragment nuclei in the cytoplasm in presence of a test substance, is an indication of mutagenic potential of the tested substance. This test is widely used for quantitative analyze a genotoxic potential of various substances. Representative images of cells with stained nuclei incubated with different testing substances are shown in the figure [Figure 4]. Analysis of fluorescence images as well as quantitative data revealed that the positive controls (cyclophosphamide and ethyl methanesulfonate) induced substantial DNA damage leading to high number of micronuclei formation while none of CD showed any sign of genotoxicity with or without metabolic activation system [Figure 4].
Cytotoxicity of inclusion complexes
The cytotoxicity studies of complexes were performed using inclusion complex batches of highest molar ratio of CD to PTX (5:1).
Figure 1: Representative differential scanning calorimetry (DSC) thermograms of free paclitaxel (PTX), β-cyclodextrins (CDs) and their mixtures and inclusion complexes with PTX; (a) – Chemical structure of PTX. (b) – Free PTX; (c) – Hydroxy propyl β-cyclodextrin (HPCD); (d) Physical mixture of HPCD and PTX (e) – Hydroxy propyl β-cyclodextrin-paclitaxel (HPCD-PTX); (f) – Methyl β-cyclodextrin (MeCD); (g) Physical mixture of MeCD and PTX; (h) – Methyl β-cyclodextrinpaclitaxel (MeCD-PTX); (i) – Sulfobutyl ether β-cyclodextrin (SBCD); (j) – Physical mixture of SBCD and PTX; (k) – Sulfobutyl ether β-cyclodextrin-paclitaxel (SBCD-PTX). A vertical bar on ordinate corresponds to 1 W/g of heat flow.
Both the fluorescence and flow cytometry studies showed that when PTX was complexed with CD, higher amounts of the drug were internalized by the cell compared to free PTX. As can be seen in the representative images, visibly higher fluorescence was observed in cells treated with CD-PTX complexes when compared with free PTX alone [Figure 6a]. The FACS analysis showed 1.5 fold higher fluorescence intensities with complexes when compared with samples of free PTX reflecting higher cellular internalization of the drug complexed with CDs [Figure 6b]. These results corroborated cytotoxicity data and showed that higher amount of PTX is available within the cell when administered as a complex compared to free drug.
Discussion
As the molecular PTX: CD ratio was increased, HPCD (in contrast to MeCD and SBCD) showed a strong linear correlation between the ratio and solubility [16]. The high entrapment efficiency of PTX partly attributed to the choice of the co-solvent method where more PTX is available to complex with CD. In co-solvent system PTXfavored higher inclusion and thus higher complexation was achieved at relatively lower molar ratios [17]. In the absence of organic solvents, the low aqueous solubility of PTX may become a rate-limiting factor restricting the entrapment efficiency. Relatively low entrapment efficiency with MeCD could be attributed to high steric hindrance of methyl substitution on CD ring affecting PTX inclusion in the cavity.
Figure 2:Representative nuclear magnetic resonance (NMR) spectra of β-cyclodextrins (CDs) and their inclusion complexes with paclitaxel (PTX). (a) – Hydroxy propyl β-cyclodextrin (HPCD); (b) – Hydroxy propyl β-cyclodextrin-paclitaxel (HPCD-PTX); (c) – Methyl β-cyclodextrin (MeCD); (d) – Methyl β-cyclodextrinpaclitaxel (MeCD-PTX); (e) – Sulfobutyl ether β-cyclodextrin (SBCD); (f) – Sulfobutyl ether β-cyclodextrin-paclitaxel (SBCD-PTX).
Figure 3:Representative X-ray diffraction spectra of free paclitaxel (PTX) and β-cyclodextrin (CD) inclusion complexes with PTX. (a) – Free PTX; (b) – Methylβ-cyclodextrin-paclitaxel (MeCD-PTX); (c) – Sulfobutyl ether β-cyclodextrin-paclitaxel (SBCD-PTX); (d) – Hydroxy propyl β-cyclodextrin-paclitaxel (HPCD-PTX).
Theoretically, if all CD molecules formed 1:1 complexes, there is no need to use higher amount of CD. However, the methods used for the preparation of such complexes are not advanced enough to take the full advantage of every single cavity in all the CD molecules. In addition, the phase solubility studies of several drugs have revealed that some drugs employ more than 1 molecule of CD per molecule of a drug, which may result in either 1:2, 1:3 or sometimes a mixture of these complexes [18]. Hence, it would be difficult to determine the Whether EP94 could protect rat hearts during reperfusion, we administrated EP94 (1 μg/kg) during the first 5 minutes of LCA reperfusion. Although EP94 was protective when given before or during ischemia, it failed to decrease infarct size when given at reperfusion in the present study. There were no noticeable effects of EP94 on reduction of infarct size when peptide was administrated in the first seconds or minutes of reperfusion (36.8 ± 1.9% and 42.6 ± 1.7% respectively versus 42.9 ± 2.7% in control group, [Figure 2] exact stoichiometric ratio of CD and PTX in the formulations.
Figure 4:Genotoxicity of β-cyclodextrins (CDs). (a) - (j) – representative images of cells stained with nuclear dye incubated with the following substances: (a) – Media (untreated cells, negative control); (b) – DMSO (negative control for the solvent); (c) – Cyclophosphamide (Cyc) with metabolic activator - S9 mix (positive control 1); (d) – Ethyl methanesulfone - EMS (positive control 2); (e) – Hydroxy propyl β-cyclodextrin (HPCD); (f) – HPCD + S9; (g) – Methyl β-cyclodextrin (MeCD); (h) – MeCD + S9; (i) – Sulfobutyl ether β-cyclodextrin (SBCD); (j) – SBCD + S9. (k) – Quantitative analysis of micronuclei formation. Means ± SD are shown. *P < 0.05 when compared with control (untreated cells). Arrows indicate micronuclei.
Figure 6:Cellular internalization of free and β-cyclodextrin – bound fluorescently labelled paclitaxel. (a) Representative light and fluorescence images of cells incubated within 3 h with substances indicated. (b) Representative flow cytometry fluorescence intensity charts. PTX – free non-bound paclitaxel; HPCD-PTX – Hydroxy propyl β-cyclodextrin-paclitaxel; MeCD-PTX – Methyl β-cyclodextrin-paclitaxel; SBCD-PTX – Sulfobutyl ether β-cyclodextrin-paclitaxel
The ability of CDs to form inclusion complexes with paclitaxel has been known for years. Though several publications cited use of HPCD, any comprehensive work of PTX complexes with either MeCD or SBCD hasn’t been reported till date [20,21]. The studies presented in the manuscript aimed to fulfill the gap by comparing PTX complexes with three different derivatives of CD.
In summary, the data obtained showed that CD complexation not only improved solubility but also facilitated cellular internalization of PTX. The in vitro genotoxicity and cytotoxicity studies confirmed safety of CDs as excipients. In the future, such PTX-CD complexes can be used via parenteral infusion as a suitable substitute obviating use of Cremophor EL. Finally, the CD-based formulation approach may help to solve problems with several other BCS class IV drugs due to combined effect on solubility and improved cellular internalization.
References
- FDA (2009) The biopharmaceutics classification system (BCS) guidance.
- Amidon GL, Lennernas H, Shah VP, Crison JR (1995) A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12: 413-420.
- Rodriguez-Antona C (2010) Pharmacogenomics of paclitaxel. Pharmacogenomics 11: 621-623.
- Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, et al. (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8: 1263-1268.
- Arbuck SG, Canetta R, Onetto N, Christian MC (1993) Current dosage and schedule issues in the development of paclitaxel (Taxol). Semin Oncol 20: 31-39.
- Gelderblom H, Verweij J, Nooter K, Sparreboom A (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37: 1590-1598.
- Biernacka J, Betlejewska-Kielak K, Witowska-Jarosz J, Klosinska-Szmurlo E, Mazurek AP (2014) Mass spectrometry and molecular modeling studies on the inclusion complexes between alendronate and beta-cyclodextrin. J Incl Phenom Macrocycl Chem 78: 437-443.
- Mazzaferro S, Bouchemal K, Gallard JF, Iorga BI, Cheron M, et al, (2011) Bivalent sequential binding of docetaxel to methyl-beta-cyclodextrin. Int J Pharm 416: 171-180.
- Yu Z, Cui M, Yan C, Song F, Liu Z, et al. (2007) Investigation of heptakis(2,6-di-O-methyl)-beta-cyclodextrin inclusion complexes with flavonoid glycosides by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 21: 683-690.
- Dordunoo SK, Burt HM (1996) Solubility and stability of taxol: effects of buffers and cyclodextrins. Int J Pharm 133: 191-201.
- Stella VJ, He Q (2008) Cyclodextrins. Toxicol Pathol 36: 30-42.
- Alcaro S, Ventura CA, Paolino D, Battaglia D, Ortuso F, et al. (2002) Preparation, characterization, molecular modeling and in vitro activity of paclitaxel-cyclodextrin complexes. Bioorg Med Chem Lett 12: 1637-1641.
- Sharma US., Balasubramanian SV, Straubinger RM (1995) Pharmaceutical and physical properties of paclitaxel (Taxol) complexes with cyclodextrins. J Pharm Sci 84: 1223-1230.
- Shah V, Taratula O, Garbuzenko OB, Patil ML, Savla R, et al. (2013) Genotoxicity of different nanocarriers: possible modifications for the delivery of nucleic acids. Curr Drug Discov Technol 10: 8-15.
- Pakunlu RI, Wang Y, Tsao W, Pozharov V, Cook TJ et al. (2004) Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery system. Cancer Res 64: 6214-6224.
- Higuchi TC (1965) Phase Solubility Techniques. Adv Anal Chem Instrum 4: 117-212.
- Hamada H, Ishihara K, Masuoka N, Mikuni K, Nakajima N (2006) Enhancement of water-solubility and bioactivity of paclitaxel using modified cyclodextrins. J Biosci Bioeng 102: 369-371.
- Loftsson T, Masson M, Brewster ME (2004) Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93: 1091-1099.
- Bouquet W, Boterberg T, Ceelen W, Pattyn P, Peeters M et al. (2009) In vitro cytotoxicity of paclitaxel/beta-cyclodextrin complexes for HIPEC. Int J Pharm 367: 148-154.
- Bouquet W, Ceelen W, Fritzinger B, Pattyn P, Peeters M, et al. (2007) Paclitaxel/beta-cyclodextrin complexes for hyperthermic peritoneal perfusion - formulation and stability. Eur J Pharm Biopharm 66: 391-397.
- Cserhati T, Forgacs E, Hollo J (1995) Interaction of taxol and other anticancer drugs with alpha-cyclodextrin. J Pharm Biomed Anal 13: 533-541.