Journal of Pediatrics & Child Care
Download PDF
Research Article
Efficacy Medical Treatment as an Alternative to surgical treatment in Management of Infantile Hypertrophic Pyloric Stenosis (IHPS)
Omar Atef Elekiabi1 , Mohamed E. Eraky1 , Loay M. Gertallah2* and Ali M. Hassanin3
- 1Department of Pediatric surgery, Zagazig University, Zagazig, Egypt
- 2Department of General surgery, Zagazig University, Zagazig, Egypt
- 3Department of Radiology, Zagazig University, Zagazig, Egypt
- Wu SF, Lin HY, Huang FK, Chen AC, Li CI, et al. (2016) Efficacy of Medical Treatment for Infantile Hypertrophic Pyloric Stenosis: A Meta-analysis. Pediatr Neonatol 57: 515-521.
- Dufour H, Fredet P (1908) La stenose hypertrophique du pylore chez le nourrisson et son traitement chirurgical. Rev Chir 37: 208-253.
- Ramstedt C (1912) Zur Operation der angeborenen Pylorusstenose. MedKlinik ;8:1702-5.
- Kawahara H, Takama Y, Yoshida H, Nakai H, Kubota A, et al. (2005) Medical treatment of infantile hypertrophic pyloric stenosis: should we always slice the “olive” ? J Pediatr Surg 40: 1848-1851.
- Corner BD (1955) Hypertrophic pyloric stenosis in infancy treated with methyl scopolamine nitrate. Arch Dis Child 30: 377-86.
- Nagita A, Yamaguchi J, Amemoto K, Yamaguchi J, Mino M, et al. (1996) Management and ultra-sonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr 23: 172-177.
- Kawahara H, Imura K, Nishikawa M, Yagi M, Kubota A, et al. (2002) Intravenous atropine treatment in infantile hypertrophic pyloric stenosis. Arch Dis Child 87: 71-74.
- Okazaki T, Yamataka A, Fujiwara T, Nishiye H, Miyano T, et al. (1994) Abnormal distribution of nerve terminals in infantile hypertrophic pyloric stenosis. J Pediatr Surg 29: 655-658.
- Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ, De Laet MH, et al. (1992) Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. N Eng J Med 327: 511-515.
- Omura N, Kashiwagi H and Aoki T (1993) Changes in gastric hormones associated with gastric outlet obstruction. An experimental study in rats. Scand J Gastroenterol 28: 568-572.
- Sherwood W, Choudhry M, Lakhoo K (2007) Infantile hypertrophic pyloric stenosis: an infectious cause? Pediatr Surg Int 23: 61-63.
- Schechter R, Torfs CP, Bateson TF (1997) The epidemiology of infantile hypertrophic pyloric stenosis. Paediatr Perinat Epidemiol 11: 407-427.
- Carter CO (1961) The inheritance of congenital pyloric stenosis. Br Med Bull 17: 251-254.
- Brown JH (2006) Atropine, scopolamine and related anti-muscarinic drugs. Arch Gen Psychiatry 63: 1121-1129.
- Kobayashi H, O’Briain DS, Puri P (1994) Selective reduction in intramuscular never supporting cells in infantile hypertrophic pyloric stenosis. J Pediatr Surg 29: 651-654.
- Mercer AE, Phillips R (2013) Question 2: can a conservative approach to the treatment of hypertrophic pyloric stenosis with atropine be considered a real alternative to surgical pyloromyotomy? Arch Dis Child 98: 474-477.
- Yamataka A, Tsukada K, Yokoyama-Laws Y, Murata M, Lane GJ, et al.(2000) Pyloromyotomy versus atropine sulfate for infantile hypertrophic pyloric stenosis. J Pediatr Surg 35: 338-342.
- Riccabona M, Weitzer C, Lindbichler F, Mayr J (2001) Sonography and color Doppler sonography for monitoring conservatively treated infantile hypertrophic pyloric stenosis. J Ultrasound Med 20: 997-1002.
- Singh UK, Kumar R, Suman S (2001) Successful management of infantile hypertrophic pyloric stenosis with atropine sulfate. Indian Pediatr 38: 1099-1105.
- Huang YC, Su BH (2004) Medical treatment with atropine sulfate for hypertrophic pyloric stenosis. Acta Paediatr Taiwan 45: 136-140.
- Sretenovic A, Smoljanic Z, Korac G, Lukac M, Krstić Z et al. (2004)Conservative treatment of hypertrophic pyloric stenosis in children. Srp Arh Celok Lek 132: 93-96.
- Singh UK, Kumar R, Prasad R (2005) Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr 42: 473-476.
- Meissner PE, Engelmann G, Troeger J, Linderkamp O, Nuetzenadel W, et al. (2006) Conservative treatment of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate does not replace pyloromyotomy. Pediatr Surg Int 22: 1021-1024.
- Lukac M, Antunovic SS, Vujovic D, Pavicevic P, Jesic M, et al. (2013) Is abandonment of nonoperative management of hypertropic pyloric stenosis warranted? Eur J Pediatr Surg 23: 80-84.
- Takeuchi M, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S, et al.(2013) Pyloromyotomy versus i.v. atropine therapy for the treatment of infantile pyloric stenosis: nationwide hospital discharge database analysis. Pediatr Int 55: 488-491.
*Address for Correspondence: Loay MG, Department of General surgery, Faculty of Medicine, Zagazig University, Zagazig, Egypt; E-mail: loayelhady@gmail.com
Citation: Elekiabi OA, Eraky ME, Loay MG, Hassanin AM. Efficacy Medical Treatment as an Alternative to surgical treatment in Management of infantile hypertrophic Pyloric Stenosis (IHPS). J Pediatr Child Care. 2018;4(1): 01.
Copyright: © 2018 Elekiabi OA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 4, Issue: 1
Submission: 03 May 2018 |Accepted: December 12, 2018 | Published: December 14, 2018
Keywords
Infantile hypertrophic pyloric stenosis; Atropine therapy; Pyloromyotomy
Abstract
Background: Background: Pyloromyotomy is considered the main surgical management for treatment of Infantile Hypertrophic Pyloric Stenosis(IHPS). Medical treatment with atropine therapy for management IHPScould be even more beneficial than open surgery and laparoscopic pyloromyotomy mainly in infants with major congenital anomaly orcomorbid conditions and in cases of refusal of patients to perform surgery to the infants.
Aims: To evaluate and assess the values and efficacy of atropineas a non-surgical alternative to pyloromyotomy in management of IHPSand to investigate the sonographic changes of the pyloric canal, thebenefits and adverse effects of atropine, with special consideration to improvement of pyloric hypertrophy.
Patients and methods: we have included 25 infants with clinical and sonographic evidences of having IHPS. We give them intravenous atropine at a dose of 0.01 mg/kg 6 times daily before feeding. When vomiting decreased and they became able to take oral formula of about 150 ml/kg/day; we gradually increased the feeding volume then we gave them oral atropine 0.02 mg/kg six times a day and then we decreased the dose was gradually and we noticed and reported improvements.
Results: we found that 20 (80%) of our included 25 infants have stopped projectile vomiting after intravenous atropine treatment in a median time of 7 days and then oral atropine intake followed for about median 44 days. We proved that there are no significant complications occurred from atropine treatment. Ultra-sonography revealed that there was a significant decrease in pyloric muscle thickness, but there was no significant shortening of the pyloric canal after termination of the medical treatment with atropine. At presentation our in included infants exhibited failure to thrive, but they were thriving at 6 months of age (p < 0.01). The remaining 5 infants were not improved with IV atropine and they required surgery 4 infants of them showed dramatic improvement of vomiting after surgery (p < 0.01).
Conclusions: in IHPS medical treatment with atropine resulted in a clinical recovery and a significant reduction in pyloric muscle thickness, so that this therapy is an effective alternative to pyloromyotomy if the length of the hospital stay and the need of continuing oral atropine medication are accepted by the parents of the infants.
Introduction
One of the most common causes of vomiting in infants is Infantile Hypertrophic Pyloric Stenosis (IHPS) and it mostly needs surgical intervention [1]. IHPS is caused by an abnormal pyloric muscle hypertrophy, and pyloromyotomy, which is primarily introduced by Dufur and Fredet then by Ramstedt in [2,3] , become the main surgical management of IHPS, as it is considered an effective line of surgical management that can be easily done with negligible complications and very rare mortality. Moreover, due to technological advancement and popularity of laparoscopic surgery even for infants and small children, laparoscopic pyloromyotomy has become a preferred line of management of IHPS particularly in western countries. However, its values and success rates are still not proved yet in comparison with open surgery [4] . Moreover, the effect of medical treatment as a less invasive procedure than laparoscopic or open surgery in management of IHPS, many previous studies have tried to manage it by medical treatment as an alternative to surgery. In 1955, Corner has tried to use methyl scopolamine nitrate as medical treatment for IHPS. Moreover, Nagita et al. [5,6] , used intravenous methyl atropine nitrate; so, recent studies pointed to the positive roles of atropine in management of IHPS. Oral atropine in small doses is not working adequately in infants as they have frequent projectile vomiting, but it was found to work if given at a high dose. Recent researchers assessed the benefits, safety and efficacy of using intravenous atropine therapy for IHPS as alternative line of management to surgery [4] .
Values of intravenous atropine in management of IHPS are still controversial and it has not gained wide acceptance due to the need of a long time of hospital stay and treatment at home. Moreover, opened or laparoscopic pyloromyotomy are considered safe and optimum lines of management of IHPS, but the negative consequences and complications of surgery cannot be ignored [7] . Patient will have an abdominal scar, so there is a small long term risk of complications due to presence of dehiscence and adhesion, additionally; expert pediatric surgeons are not always easily available. So we have performed such study to compare the outcomes, benefits, safety, and efficacy and cost-effectiveness of medical treatment using intravenous atropine as a safe alternative to surgery. We tried in this study to confirm that medical treatment is better that performing pyloromyotomy mainly in infants with major congenital anomaly or co-morbid conditions and in cases of refusal of patients to perform surgery to the infants.
Aims
To evaluate and assess the values and efficacy of atropine as a non-surgical alternative to pyloromyotomy in management of IHPS and to investigate the sonographic changes of the pyloric canal, the benefits and adverse effects of atropine, with special consideration to improvement of pyloric hypertrophy.
Patients and Methods
Study design
A. This is a prospective cohort study that was done in Pediatric Surgery department, Faculty of Medicine Zagazig University and department of Radiology Faculty of Medicine Zagazig University.
B. Approval was obtained from the IRB committee in Faculty of Medicine Zagazig University, and a written informed consent was obtained from the patients’ guardians.
C. We have included 25 infants with clinical and/or radiological evidence of IHPS that were admitted to Pediatric Surgery department, in the period September 2016 to September 2018.
Inclusion criteria of patients included in our study:
A. All infants have the following diagnostic criteria for IHPS: (a) repeated attacks of projectile vomiting more than 2 times daily (b) Ultrasonic evidence of gastric outlet obstruction, narrow and long pyloric canal; (c) pyloric canal of more than 15 mm in length and pyloric muscle more than 4 mm in thickening on ultrasonography.( (Figure 1A).
B. Refusal of parents to perform pyloromyotomy and prefer trying medical treatment after informing them with the costs and duration of medical therapy.
C. Infants with congenital anomaly for which surgery might be risky to them.
Detailed medical treatment
• Intravenous atropine was given to the patients at a dose of 0.01mg/ kg 6 times a day 10 minutes before the feeding.
• We monitored the infant heart rate in the first days of starting atropine IV infusion by electrocardiography.
• We have started giving the infants the oral feeding at a volume of 10 ml formula, six times daily.
• We have then increased the volume a day after a day until infants were able to tolerate 150 ml/kg/day, except if vomiting occurred more than two times daily.
• We have given the infants oral atropine in a dose of 0.02 mg/kg orally six times a day before feeding when infants were able to tolerate the full formula volume without vomiting more than two times daily.
• We have discharged infants from the hospital when vomiting was stopped or controlled with oral atropine.
• When the infants were free of vomiting and showed a progressive increase in weight, we decreased atropine in 3steps (0.12 mg/kg/ daily, 0.06 mg/kg/ daily, 0.03 mg/kg/ daily).
• We restarted oral atropine again if infants vomited more than twice daily for three days after discontinuation of atropine treatment.
• We have recorded the ultra-sonographic evaluations at time of presentation, at 3 weeks and 6 months after termination of administration of oral atropine and at 1 year of age.
• We have performed the ultra-sonography while the infants were fasted for three hours before examination.
• We have measured the pyloric muscle thickness, the pyloric canal length and transpyloric flow of gastric contents.
• We have assessed the physical development of the infants by examination of serial changes in their body weight at presentation and at the ages of three months, six months.
• Finally we have considered IV atropine treatment unsuccessful if the infants have failed to tolerate half of the full feeding volume within a week or the full feeding volume within two weeks.
• The five infants who failed to improve by medical treatment were managed by pyloromyotomy.
• Surgery was performed with pyloromyotomy which was done by using either the supra-umbilical skin-fold incision or the right upper quadrant incision.
Technique of operative management
• General anesthesia is used.
• Infant is in supine position.
• Naso-gastric tube is inserted.
• Right upper quadrant transverse incision is used.
• Cutting of external and internal oblique muscles.
• Splitting of transverse abdominis muscle.
• Peritoneum is opened, body of the stomach is identified and held out of the wound.
• If stomach is not clearly identifiable, then 30 ml of air is insufflated in to stomach to make it visible.
• Greater curvature of stomach is held and drowns towards left with traction.
• Mass extends distally up to pyloric vein of mayo.
• Middle of pylorus and mass on the anterior aspect is incised longitudinally for its entire length and thickness.
• Care should be taken not to injure the mucosa
• Adequate hemostasis was done.
• Closure of abdomen in layers without drain.
• Four patients of the five patients that were managed surgically showed dramatic improvement of vomiting.
• We have conducted peripheral blood cell counts, electrolytes, and biochemical examination before surgical intervention or medical treatment as routine investigations.
• We have followed our successfully treated infants were followed up for 2 years.
Statistical Analysis
Data were analyzed using Statistical Package for Social Science for windows version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were checked for normality by using Shapiro-Wilk test. Independent Student t-test was used to compare two groups of normally distributed data, while Mann-Whitney U was used for non-normally distributed data. Percent of categorical variables were compared using Chi-square test. All tests were two sided. P-value <0.05 was considered statistically significant.
Results
Content analysis
The analysis strategy focused on both summative content analysis and interpretation in which as many ideas and details were noted and initially coded. These broad ideas began with a brief examination of the first few responses from students from each Grade (Table 1). This analysis progressed toward more focused coding with remaining responses. Constant comparison strategy was used to compare initial data with data, data with concepts, and between concepts until a select set of content was observed to be central to student responses as a whole. Two researchers (DMH and SMH) developed coding systems individually and through team discussions to ensure internal validity of inter-rater procedures. Any discrepancies were discussed until full agreement was reached.
n = Total number of patients; Quantitative data were expressed as mean ± SD (standard deviation); Qualitative data were expressed as number (percentage).
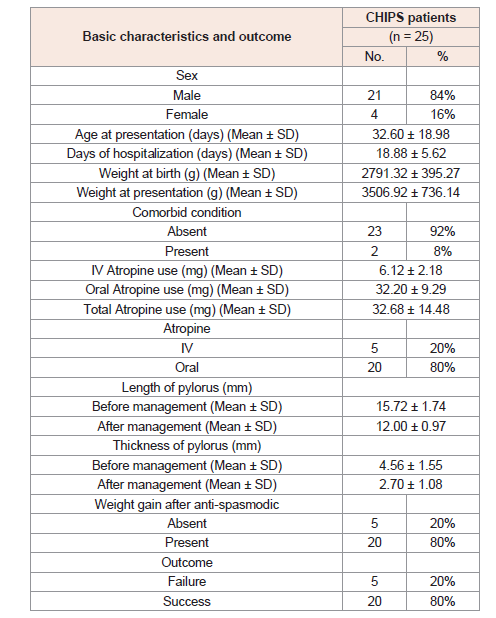
Basic characteristics and outcome of all the studied CHIPS patients following conservative management are included in (Table 1).
Patients are divided into 21 (84%) males and 4 (16%) females.
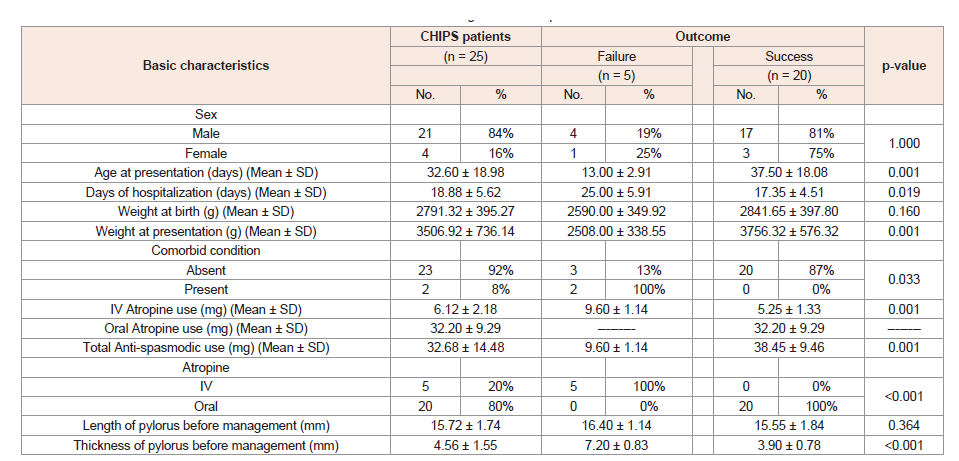
Demonstrated Factors which determine technical and clinical success of conservative management of the studied 25 CHIPS patients (Table 2).
Table 2: Factors determine technical and clinical success of conservative management of our patients.
n = Total number of patients in each group; Quantitative data were expressed as mean ± SD; Qualitative data were expressed as number (percentage); MannWhitney U test; ‡ Chi-square test; p-value< 0.05 is significant.
Of the 20 patients, 15 experienced little vomiting three weeks after completion of oral atropine administration.
The remaining 5 occasionally vomited once or twice a day three weeks after completion of oral atropine, but rarely vomited three and six months after completion.
Of the 25 patients, 20 (80%) became free from projectile vomiting during atropine treatment.
The remaining 5 (20%) required surgery after 9 and 18 days of intravenous atropine.
Weight SDSs showed significant changes during atropine treatment (p < 0.01).
There was a significant (p < 0.01) increase in weight at 6 months of age, at 1 year and 2 years of age compared with that at presentation.
Ultra-sonography showed significant (p < 0.01) changes during atropine treatment.
There are serial changes in pyloric muscle thickness and pyloric canal length.
Pyloric muscle thickness decreased significantly from 5 (4-6) mm at presentation to 3 (2-5) mm three weeks after completion of oral atropine (p < 0.001).
Pyloric canal length was 19 (15-25) mm at presentation and 15 (12-20) mm three weeks after completion of oral atropine but the change was statistically not significant.
Facial flushing was not reported during treatment. The resting heart rate remained less than160 beats/min.
Operative management of Patients with failed medical management
A supraumbilical skin-fold incision was used on 3 patients and aright upper quadrant incision on 2 patients. Of 25 patients, 20 who were successfully treated with atropineand 5 who were surgically treated were reviewed at the age of 1 year.
Four patients of the five patients that were managed surgically showed dramatic improvement of vomiting.
The patients did not show any symptoms related to IHPS.
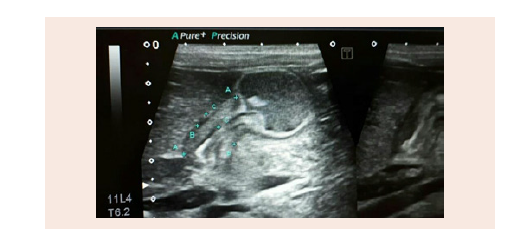
Ultrasound of the pyloric region (A) shows elongation of the pyloric canal and thickening of its wall muscles (B) The length of the pyloric canal is 18 mm The width about 15 mm and the thickness of the muscle wall is 6 mm (Figure 1B)
Discussion
Medical treatment of IHPS using IV then oral atropine has been emerged as an alternative optional management [1,7]. Nagita et al. [6] have reported a high success rate in cases of using IV atropine treatment which was gradually increased until vomiting was controlled, so they proved that there was a wide variation in the final dose of administered atropine between patients, but the included sample size which in previous studies was small. Due to the importance of such issue, Wu et al. [1] have reviewed all the previously published studies in their meta-analysis and have tried to assess the course and outcome of IHPS patients that were managed with atropine.
In our study we have proved that atropine could be used as a successful medical therapy in cases of IHPS and it will be better and less invasive than pyloromyotomy in certain cases, our results were similar to final findings of Wu a, et al. [1], meta-analysis who proved that either oral or IV atropine was an effective line treatment for IHPS in most studies which are included in their meta-analysis.
There are several mechanisms which are responsible for pathogenesis of IHPS including impaired functions of acetylcholine and/or muscarinic receptors, genetic basis decreased nitric oxide synthase activity, elevated prostaglandin and gastrin levels and infections [8-13].
The mechanisms of action of atropine sulfate in treatment of IHPS involve potent anti-cholinergic and antimuscarinic effects which decrease peristaltic contractions by causing relaxation of the pyloric smooth muscles [14].
The effective administered dose of atropine varies widely, due to the differences in the muscarinic receptor sensitivity of the muscles, disturbed blood flow to the pylorus secondary to pyloric spasm, variations in drug clearance, absence nitric oxide synthase, and poor circular musculature of the pylorus innervations [8,9,14,15]
The activity of IV atropine is considered two to three times more than that of the oral atropine, with a faster response to the effective dose of IV atropine. However, IV atropine is associated with side effects, like flushing and transient tachycardia.
Oral atropine has fewer side effects but it is absorbed from the intestines, diluted with gastric fluids and delayed emptying of the stomach in cases of IHPS might prevent its desired therapeutic effects. In our study, our patients were receiving IV atropine followed by oral atropine that induced remission of IHPS. Additionally, we have found that initial IV atropine is effective more than oral atropine for management of IHPS which was similar to results of Wu et al. [1], meta-analysis. Similarly, reported a higher success rate for conservative therapy using atropine in patients with IHPS [16]. Also our results were similar to previous studies [4,6,7,17-23].
Results of most previous studies showed that vomiting was markedly decreased within seven days, with improvement and normalization of the pyloric canal length in infants who are treated with atropine which was in line with our results.
Singh et al 2001 have reported that vomiting has disappeared after medical treatment with atropine in one to three days, four to seven days, nine to twelve days in patients with mild, moderate, and severe IHPS, respectively.
Kawahara et al 2005 have found a statistically significant increase in body weight at the time of presentation when they compared patients with successful and unsuccessful atropine therapy which was similar to our results. But, Meissner et al 2006 have not found any differences in body weight at time of admission between these two groups. We have reported pyloric canal normalization after termination of medical treatment with atropine which is evaluated by serial ultra-sonography, Similarly, Nagita et al. 1996, reported that time to pyloric muscle thickness occurrence of normalization of ranged from 4 to 12 months. Yamataka et al. 2000 reported that time to normalization of pyloric muscle thickness ranged from two to 3.4 months, with no significant difference with the pyloromyotomy group. Kawahara et al 2002 reported that the thickness of the pyloric muscle significantly decreased from 5 mm at time of primary presentation to 3 mm 3 weeks after termination of oral atropine therapy. Huang et al., 2004 proved that patients receiving oral atropine showed pyloric canal normalization in 35 to 47 days. Results of all these previous reports, proved that time to pyloric muscle normalization ranged from 5 weeks to 15 months for infants with IHPS who were treated with atropine which was in line with our results.
The results of Wu et al. [1], meta-analysis proved that there were no severe adverse effects or complications related to the use of oral atropine. Our results were similar to management of Kawahara et al. [4], Huang et al. [20], Kawahara et al. [7], who used IV atropine for IHPS with a hospital stay of 13 to 14 days.
Moreover, results of three studies proved that there was a prolonged hospital stay in the group which was managed by IV atropine [23-25].
Regarding financial affairs of the patients, Yamataka et al. [17], stated that expenses were lower in the group which was managed by atropine than in the pyloromyotomy group, but Kawahara et al. [4], and Takeuchi et al. [23], found different results that the costs in medically treated cases was not different from cases treated by pyloromyotomy due to the prolonged hospitalization in the medically managed cases.
Most of the studies which are included in Wu et al. [1], metaanalysis proved similar results to us that there a strong tendency for successful medical treatment, but Riccabona et al. found different results from ours that they had only 32% success rate for medical treatment with IVatropine [18]. In Riccabona et al. study, there was a statistically significant difference in pyloric length of 15 mm in patients with successful medical treatment versus 18 mm in patients who underwent surgery which explain the lower rate of success in patients who received medical treatment.
Conclusion
IHPS is a very common cause of vomiting in infants, medical treatment of IHPS patients with oral or IV atropine is found to be a good alternative to pyloromyotomy, mainly in infants with comorbid comorbid conditions or when parents refused to let their infants undergo surgery.
Pyloromyotomy is a very effective surgical treatment for IHPS which has a shorter length of stay in hospital than medical treatment. But future complications of surgery could not be expected and so, surgical treatment with pyloromyotomy is needed in infants with marked dehydration and hematemesis, if medical treatment failed.