Journal of Ocular Biology
Effect of a Soluble Epoxide Hydrolase Inhibitor, UC1728, on LPS-Induced Uveitis in the Rabbit
Gillian J. McLellan1,3,5, Zeynep Aktas1,6, Elizabeth Hennes-Beean1, Aaron W. Kolb1, Inna V. Larsen1, Emily J.Schmitz1, Hilary R. Clausius1, Jun Yang7, Sung Hee Hwang7, Christophe Morisseau7, Bora Inceoglu7, Bruce D. Hammock7, Zoraya A. Feranthy1 and Curtis R.Brandt1,2,4,5*
- 1Department of Ophthalmology and Visual Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Wisconsin, USA
- 2Department of Medical Microbiology and Immunology, School of Medicine and Public Health, University of Wisconsin-Madison, Wisconsin, USA
- 3Department of Surgical Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, Wisconsin, USA
- 4Comparative Ophthalmic Research Laboratories, School of Veterinary Medicine, University of Wisconsin-Madison, Wisconsin, USA
- 5Department of Ophthalmology and Visual Sciences, McPherson Eye Research Institute, University of Wisconsin-Madison, Wisconsin, USA
- 6Department of Surgical Sciences, Gazi University, Turkey
- 7Department of Entomology and Comprehensive Cancer Center, University of California, Davis, CA 95616, USA
*Address for Correspondence: Curtis R. Brandt, PhD, Department of Ophthalmology and Visual Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, 550 Bardeen, 1300 University Avenue, Madison, Wisconsin 53706, USA, Tel: 608-262-8054; Fax: 608-262-0479; E-mail: crbrandt@wisc.edu
Citation: Gillian J, Aktas MZ, Hennes-Beean E, Aaron W, Inna KV, et al. Effect of a Soluble Epoxide Hydrolase Inhibitor, UC1728, on LPS-Induced Uveitis in the Rabbit. J Ocular Biol. 2016; 4(1): 7.
Copyright © 2016 McLellan GJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 4, Issue: 1
Submission: 23 December 2015 | Accepted: 07 January 2016 | Published: 12 January 2016
Reviewed & Approved by: Dr. Yongdong Zhou, Assistant Professor (Research) of Ophthalmology and Neuroscience Center of Excellence, Louisiana State University Health Sciences Center, USA
Abstract
Cytochrome P450 epoxygenase isozymes convert free arachidonic acid into eicosanoids named epoxyeicosatrienoic acids (EETs) that have roles in regulating inflammation. EETs are rapidly converted to dihydroxyeicosatrienoic acids (DiHETs) by soluble epoxide hydrolase (sEH). Little is known about the potential role of these metabolites in uveitis, but conversion of EETs to DiHETs could contribute to the inflammation. We tested a potent and orally available inhibitor of sEH for its ability to reduce ocular inflammation in a rabbit LPS-induced model of uveitis. Rabbits were treated by subcutaneous injection with the sEH inhibitor (UC1728, 3 mg/kg), or the vehicle control (PEG400) and uveitis was assessed at 6, 24 and 48 h post-intracameral LPS injection using a modified Hackett-McDonald scoring system. Eyes treated by intra-cameral injection of PBS, or by aseptic preparation served as further controls. Signs of inflammation in this model were mild and transient. Treatment with UC1728 did not significantly reduce inflammation compared to animals treated with the PEG400 vehicle. Blood levels of UC1728 were a thousand fold higher than the in vitro determined inhibitory potency (IC50) of the compound suggesting a significant degree of inhibition of sEH in the rabbit. The lack of efficacy suggests that sEH or its substrates the EETs may not be involved in mediating inflammation in this model of uveitis.
Keywords
UC1728; Uveitis, Cytochrome P450; Soluble epoxide hydrolase inhibitor; Inflammation
Abbreviations
AA: Arachidonic Acid; DiHETS: DiHydroxyeicosatrienoic Acids; DiHOMEs: Dihydroxy Octadecamoenoate Esters; EETs: Epoxyeicosatrienoic Acids; EpOME: Monoepoxide derivatives of linoleic acid); HETEs: Mono-hydroxy-eicosatetraenoic Acids; SC: Subcutaneously; sEH: Soluble Epoxide hydrolase; TLR: Toll Like Receptor; UC1728: trans-4-{4-[3-(4-trifluormethoxy-phenyyl)- ureido]-cyclohexyloxy}-benzoic acid; VEGF: Vascular Endothelial Growth Factor
Introduction
Arachidonic acid (AA) metabolites play critical roles in a number of physiological processes, including pro-and anti-inflammatory activities, peripheral sensitization to pain, regulation of kidney function and blood pressure, protection against oxidative injury in the heart, regulation of platelet activity, and cancer [1-6]. Arachidonic acid, released from cellular membranes by phospholipase A2, is the substrate for three different pathways generating functional metabolites. Cyclooxygenases and prostaglandin synthases convert AA into prostaglandins, thromboxane, and prostacyclins. Lipoxygenases convert AA into leukotrienes, endogenous mono-hydroxyeicosatetraenoic acids (HETEs) and lipoxins; and cytochrome P450 epoxygenases convert AA into epoxyeicosatrienoic acids (EETs). The cytochrome P450 epoxygenase-derived EETs have anti-inflammatory properties and are also analgesic and anti-convulsant lipid mediators [3,5,7-9]. These bioactive lipids are degraded by the soluble epoxide hydrolase (sEH) and converted into dihydroxyeicosatrienoic acids(DiHETs) which are thought to be pro-inflammatory. Inhibition of sEH therefore stabilizes EETs by preventing or delaying their conversion into pro-inflammatory DiHETs and preserves EETs that display anti-inflammatory effects in models of systemic inflammation such as the mouse LPS induced sepsis model.
Members of the cytochrome P450 A and B subfamilies are present in the eye, particularly in ciliary body epithelium and corneal epithelium, and can be induced by exposure to various chemicals, hypoxia and inflammatory stimuli [10-20]. Evidence that cytochrome P450 isoforms have physiological roles in the eye, come from several studies. Mutations in CYP1B/1 are causative for Primary Congenital Glaucoma (reviewed in [21]), inhibition of CYP450 by stannous chloride reduced the inflammatory response in a closed eye contact lens rabbit model [22], and administration of siRNA targeting CYP4B1 inhibited corneal neovascularization in a suture-induced rabbit model by reducing production of 12-HETrE and inhibiting vascular endothelial growth factor (VEGF) expression [23]. Thus, CYP450 enzymes play important roles in regulating inflammatory and other responses in the eye.
Soluble epoxide hydrolase has been a target for drug development for some time and a large number of compounds have been tested in various models [3,7,24-28]. DiHETs have largely been found inactive or less active than EETs in assays that used these molecules barring few exceptions [29]. The finding that linoleic metabolites, DiHOMEs (dihydroxy octadecamoenoate esters), are more toxic to the lung than their corresponding EpOME (monoepoxide derivatives of linoleic acid) precursors suggest that they may be pro-inflammatory [30].
Early sEH inhibitors suffered from poor solubility and less than ideal pharmacokinetics. More recently, orally available inhibitors with improved potency have been synthesized [31]. One compound, trans-4-{4-[3-(4-trifluormethoxy-phenyyl)-ureido]-cyclohexyloxy}- benzoic acid (UC1728) is a potential inhibitor of rabbit sEH with an IC50 of 2.0 nM. UC1728 also has a favorable pharmacokinetic profile with peak serum levels (320 nM) in mice given a dose of 1 mg/kg, occurring between 5 and 7 h post-administration with a halflife of 15 to 20 h. It was successful in reducing the severe pain and inflammation associated with laminitis in horses [32]. Thus UC1728 is an excellent “probe” to understand the roles of sEH and EETs and a potential candidate for further drug development.
Uveitis encompasses a number of ocular inflammatory conditions and accounts for 5-20% of blindness in the USA and Europe. In developing countries uveitis can account for as much as 25% of legal blindness [33,34]. Corticosteroids are commonly used to treat uveitis but not all patients respond. Immunosuppressive drugs such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate are used as second line drugs. More recently, inhibitors that target TNF-α, such as infliximab, etanercept, and adalimumab, have been effective in patients that are refractory to other treatments [35]. Several of these therapeutic agents have unfavorable side effects or toxicity and carry the potential for serious adverse effects, thus additional therapeutic agents are needed to effectively treat uveitis. Three basic types of animal models have been used to test therapeutic agents for uveitis. One acute model involves the intra-vitreal or intracameralinjection of bacterial endotoxin [36,37]. Another acute model involves immunizing animals and then injecting the antigen into the anterior chamber [38]. A chronic model involves immunizing animals with interphotoreceptor retinoid-binding protein (IRBP) or visual arrestin [39]. The chronic models mimic an autoimmunemediated mechanism with T-cell involvement [35,40].
Inhibitors of the sEH have been shown to be anti-inflammatory in numerous models of acute inflammation, most prominently LPS induced systemic inflammation [41,42]. In this model inhibition of sEH increases survival at 24 h, reverses hypotension, decreases pain and decreases the levels of pro-inflammatory AA metabolites. Therefore the goal of this study was to test the hypothesis that UC1728 will be efficacious in reducing the acute inflammation induced by endotoxin in the rabbit anterior chamber model of uveitis. We found that UC1728 did not significantly reduce the severity of aqueous cell and flare or iris congestion. Mean blood level of UC1728 was 2070±196 nM 48 h after injection of UC1728 which is about a thousand fold above the in vitro IC50 on rabbit sEH arguing the lack of effect is not due to low drug levels. These results suggest that sEH, and by inference EETs and DiHETs do not play a significant role in the acute inflammation induced by endotoxin injection into the anterior chamber of rabbit eyes.
Methods
Synthesis and potency determination of sEH inhibitors
Inhibitors of sEH were synthesized, purified and characterized in house. For UC1728 the method of Hwang et al. was followed [31]. For the synthesis of UC1709 and related compounds, the methods of Tsai et al. were followed [43]. For the synthesis of UC1770, the method of Rose et al. was followed [44]. For the determination of in vitro potency of these inhibitors native sEH protein from rabbit liver cytosolic fraction was utilized. Protein quantification was done using the Pierce BCA assay. The potency of all inhibitors was tested using [3H]-t-DPPO as the substrate according to published methods [45,46][45,46]. For each IC50 determination five different concentrations of inhibitors were each tested three times. The concentration of sEH inhibitor that inhibited half of the enzyme activity is reported as the IC50 [45].
Animals
Eighteen male SPF New Zealand White Rabbits (2.6-3.2 Kg) were obtained from Harlan (Indianapolis, IN) and randomly assigned to 3 groups of 6 rabbits each. Rabbits assigned to group 1 had 20 μl sterile PBS intracamerally injected in the right eye (negative control) and all other rabbits received 100 ng LPS in 20 μl of PBS [37,47-50]. Groups 2 and 3 were treated with anti-inflammatory drug or vehicle once daily on the following schedule: 24 h prior to intra-cameral LPS or PBS injection, the day of injection and 24 h post-injection. Group 2 received UC1728 in PEG400 (3 mg/kg, SC) and Group 3 received PEG400 vehicle only (0.9 mL, subcutaneously, (SC)). To limit postprocedural discomfort, systemic buprenorphine (0.03 mg/kg SC, Reckitt-Benckiser) was administered immediately prior to returning rabbits to their cages upon recovery from anesthesia, then every 6-12 h for the first 24 h and as needed for the duration of the study. Animal research followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; NIH Guidelines for Care and Use of Animals, and was approved by the UW-Madison Institutional Animal Care and Use Committee.
Induction of uveitis
Uveitis was induced by intracameral injection of 100 ng of E. coli LPS (Sigma #L4391, St. Louis, MO) in 20 μl PBS (Mediatech, Manassas, VA) into the right eye using a 29 gauge needle, while the rabbits were anesthetized with ketamine HCl (25 mg/kg, Ft. Dodge) and xylazine (2 mg/kg, Lloyd) IM. Topical proparacaine (0.5%, Akorn, Lake Forest, IL) was applied to the ocular surface prior to intraocular injections. The ocular surface was prepared for the injection procedure using a dilute solution of 5% Povidone iodine (Aurora Pharmaceutical, Northfield, MN) in 0.9% saline (Phoenix, Burlingame, CA). During anesthesia (preparation and recovery) the cornea was protected from drying by irrigation with Balanced Salt Solution (Akorn) or, following intraocular injection, the application of ocular lubricant (Refresh Tears, Allergan) or Bacitracin-Polymixin B Preservative Free Ophthalmic Ointment (Akorn). The left eye received all the pre- and post- injection treatments, but not the LPS or PBS injection and thus was used as a procedural control for ocular preparation.
Clinical examination and scoring of inflammation
The rabbits were examined by an experienced board-certified veterinary ophthalmologist who was masked to their treatment group assignment, by slit lamp biomicroscopy (PSL Classic, Keeler, Broomall, PA) and indirect ophthalmoscopy prior to LPS injection (baseline) and then at 6 h, 24 h and 48 h post-injection. For examination of the posterior segment, the eye was dilated with topical tropicamide drops (1%, Akorn). A modification of the Hackett-McDonald scoring system, which has been previously used by members of our group, was used in this study (Supplementary Table 1) [51-53]. This scoring system has been widely used in rabbits, with modifications to include intraocular findings such as aqueous cell and flare [37] that have been used extensively by our group in toxicological studies, including those associated with intraocular inflammation, in which it has been sensitive in discriminating between subtle degrees of iris congestion, aqueous and vitreous cell and flare. In some rabbits, topical fluorescein staining of the eye was also conducted (Ful-Glo strips, Akorn, in balanced salt solution), when corneal epithelial defects were observed or suspected. In order to minimize invasive procedures, we did not sample the aqueous for drug and protein concentrations at each time point, as sampling itself results in increased protein and could allow additional drug to enter the eye.
Sampling
At 48 h post LPS injection, the rabbits were anesthetized with ketamine/xylazine prior to euthanasia by intravenous administration of sodium pentobarbital (Beuthanasia, Schering Plough/Merck, Kenilworth, NJ). Blood was collected, allowed to clot, and then centrifuged at 15,000 rpm for 5 min using an Eppendorf 5424 microfuge, and serum was collected and stored at -20 °C for analysis.
LC/MS/MS analysis for UC1728 serum concentrations
The liquid chromatography system used for analysis was an Agilent 1200 SL liquid chromatography series (Agilent, Foster City, CA). The auto sampler was kept at 4 °C. Liquid chromatography was performed on a Supelco Ascentis Express C18 HPLC 5 cm×2.1 mm, 2.7 um column (Sigma). The column was connected to a 4000 QTrap tandem mass spectrometer (Applied Biosystems/Life Technologies, Carlsbad, CA) equipped with an electrospray source (Turbo V). The instrument was operated in negative selective reaction monitoring (SRM) mode. Individual analyte standards were infused into the mass spectrometer and SRM transitions and source parameters were optimized for UC1728. The SRM transition for UC1728 was 437.2/137.1; the transition for internal standard CUDA was 339.2/214.3.
Statistical Analysis
Statistical analysis was carried out using Sigma Plot 11 (Systat Software, Inc., San Jose, CA) using the Kruskal-Wallis ANOVA on Ranks test. Differences were deemed to be significant if the p value was < 0.05.
Results
Selection of the sEH inhibitor
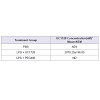
Several thousand sEH inhibitors have been synthesized to date [3] and the inhibitory potency of sEH inhibitors vary for each species. Therefore, we first determined the IC50 values of three inhibitors using a rabbit liver cytosolic extract. Of the three sEH inhibitors tested, the inhibitor UC1728 was the most potent with an IC50 value of 2 nM (Table 1) and was used for all subsequent studies.
Clinical evaluation
Baseline examinations conducted 24 h prior to endotoxinadministration revealed normal values in all subjects. Of the several parameters evaluated in the modified Hackett-McDonald scoring system, three: pupillary light reflex (miosis), conjunctival congestion, and iris congestion were consistently increased in LPS-injected eyes as markers for LPS-induced uveitis in the rabbits. Other ocular signs associated with uveitis, including anterior chamber flare, cell and fibrin, were inconsistently observed in LPS-treated eyes. Note that fibrin is inconsistent in this model and may be independent of endotoxin concentration [37]. Figure 1 panels A, B, and C shows the scores for pupillary light reflex, conjunctival and iris congestion in LPS and PBS-treated eyes. Miotic, fixed pupils were observed in the treated eyes of both groups receiving LPS injections but the difference in pupillary light reflex scores was only significantly increased in the UC1728/LPS treated group relative to the PBS group at 6 h postinjection (1A). For conjunctival congestion, the scores for the groups receiving LPS were not significantly different from each other at any time, regardless of whether animals were treated with UC1728 or vehicle (1B). The only significant difference for conjunctival congestion was between the LPS/UC1728 group and PBS group at 24 h post-treatment. Iris congestion scores were significantly higher in eyes that received LPS compared to PBS injected eyes at 6 and 24 h post-injection (1C) but this difference was no longer statistically significant at 48 h. There were no significant differences in the scores between the LPS/UC1728, and LPS/PEG400 vehicle -treated groups at any time
We also compared aggregate scores (obtained from addition of all scoring parameters) and these are shown in Figure 2. This figure also includes the control eyes which received the surgical preparation only (no LPS injection). At 6 h after injection, eyes that received surgicalpreparation only demonstrated conjunctival congestion, with or without accompanying chemosis or ocular discharge, but these changes were mild with scores limited to 1+ for these parameters and aggregate scores that were approximately half of the aggregate scores for the LPS-treated eyes. This reaction in the preparation-only eyes diminished rapidly and was minimal by 24 h post-initiation and scores returned to baseline, normal values by 48 h. At 6 h post-LPS injection, there were no significant differences in the aggregate scores between the LPS-treated groups. At 24 h post-initiation the aggregate scores for PBS treated eyes were significantly lower than scores for the eyes injected with LPS.
Overall, these results indicate that endotoxin induced uveitis in rabbits following intracameral injection of LPS is mild and transient and that treatment with the soluble epoxide hydrolase inhibitor UC1728 had little, if any, clinical efficacy in reducing LPS-induced inflammation compared to treatment with PEG400 vehicle control
Serum concentrations of UC1728 at 48 hours post-treatment
To determine if therapeutic concentrations of UC1728 were likely to have been achieved we measured inhibitor concentrations in serum samples taken 48 h post-treatment at the time of euthanasia (Table 2). The LC-MS/MS method used for UC1728 has a limit of quantification of 0.2 nM [54]. The mean serum concentration of UC1728 in Group 2 rabbits was 2070±196 nM of UC1728, which is approximately 1000-fold higher than the in vitro IC50 potency value for this inhibitor (Table 1). In all other groups UC1728 was undetectable as expected
Conclusion
Arachidonic acid metabolites have been shown to play important roles in a number of inflammatory processes and a number of arachidonic acid metabolizing enzymes are present in ocular tissues. For uveitis, there is little information available on whether EETs or metabolites such as DiHETs resulting from sEH activity, play a role but the availability of specific inhibitors makes it possible to assess the potential role of these pathways in ocular inflammation. In this work, we assessed the potential anti-inflammatory effect of an inhibitor of soluble epoxide hydrolase in a standard rabbit acute uveitis model and the results indicate that inhibition of the sEH enzyme neither enhanced nor reduced the severity of the inflammation.
The mild, transient and inconsistent nature of signs of inflammation in this particular uveitis model may have limited the ability to detect a subtle anti-inflammatory effect of UC1728. Laser flare photometry provides objective, sensitive and reproducible quantification of aqueous humor protein content and cell in clinical patients and animal models and was considered as an alternative strategy [55]. However, this method is not without limitations and there is considerable variability in values obtained from normal individuals. Degree of pupil dilation has been shown to impact values obtained by photometry and this could have confounded quantification of flare in subjects with variable degrees of mitosis in our study. A previous report comparing clinical grading of aqueous flare with aqueous flare photometry in an a rabbit endotoxin-induced uveitis model concluded that clinical slit-lamp biomicroscopy scores correlated with aqueous flare photometry values at lower grades, consistent with those observed in the current study, and indicated comparable sensitivity between these two methods for detecting subtle signs of uveitis in this model [37].
Another possible explanation for the lack of effect of UC1728 is that drug concentrations did not reach inhibitory levels. We quantified inhibitor levels in the serum of the rabbits and found the mean concentration was approximately 1,000-fold higher than the IC50 value, thus is it unlikely that this explains the lack of effect. Another possibility is that the inhibitor did not reach high enough concentrations in the eye, although the free inhibitor levels in blood, multiple administrations and observable breakdown in the bloodaqueous barrier observed in LPS-treated eyes make this less likely. A third possibility is that the anti-inflammatory effects of EETs and sEH are site and tissue selective. A major mechanism of action for EETs in reducing inflammation is postulated to be disruption of NFκ-B signaling [7]. These effects were initially observed in endothelial cells and later in cardiomyocytes [7,56]. The uveitis model induced by LPS similarly activates a Toll Like Receptor (TLR4) initiated signaling cascade. However, the specific mechanism initiated by LPS through TLR4 in the eye may not be identical to other tissues. Therefore target(s) of EETs or mechanisms mediated by EETs may not exist or may not interact with the same downstream signaling pathways as in endothelial cells. Consistent with this idea, Fife et al. did not observe anti-inflammatory effects in the liver tissue of mice systemically treated with LPS and sEH inhibitor [57]. Furthermore in that study the whole body knockout sEH-/- mice also did not display an antiinflammatory profile in liver tissues. However, with both chemical and genetic ablation, the plasma levels of anti- and pro-inflammatory eicosanoids were modulated in a manner that would be expected from inhibition of sEH.
It has previously been shown that DiHETs display proinflammatory effects by increasing the secretion of monocyte chemoattractant protein-1 (MCP-1) and inducing monocyte chemotaxis both in vitro and in vivo [58]. Therefore, one mechanism for the anti-inflammatory effects of inhibitors of sEH may be through reducing the levels of DiHETs and thus decreasing cellular infiltration at the site of inflammation. These observations may partially explain the lack of effect we saw with UC1728, since cellular infiltration was not a major feature seen in this acute LPS-induced model of inflammation.
A final possible explanation for lack of efficacy in this model is that sEH inhibitors only preserve epoxy fatty acids and do not increase their production. Thus, the concentration and rate of production of epoxy fatty acids, including EETs, may not have been sufficiently high in this model, such that their stabilization with sEH inhibitors would not have resulted in levels sufficient to prevent uveitis. This hypothesis could be tested by applying EETs and EDPs to the eye.
Although we found that inhibition of sEH did not affect LPS induced uveitis, we cannot conclude that sEH plays no role in uveitis pathophysiology. The LPS model has a very specific and restricted mechanism; activation of TLR4, which results in the downstream activation of NFκB [59], thus this model may not be appropriate for the evaluation of sEH inhibitors. It is possible that sEH may be involved in some forms of uveitis that currently available animal models do not mimic. Overexpression of sEH using gene delivery strategies or the analysis of clinical samples for the presence of sEH metabolic products might be informative as to the potential involvement of sEH and its substrates and metabolites in uveitis. Although our results showed a lack of effect, this work provides important information about the potential role, or lack thereof, of EETs and sEH metabolites in a widely used model of ocular inflammation.
Acknowledgements
This work was supported by the Comparative Ophthalmic Research Laboratories (CORL); a Vision Research Core Grant (P30EY016665), a career development award to GJM (K08EY018609) and an unrestricted grant from Research to Prevent Blindness, Inc. to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison. Partial support was also provided by NIEHS grant R01 ES002710 to BDH and the West Coast Comprehensive Metabolomics Resources Core NIH/NIDDK U24 DK097154 (BDH).
References
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA (2009) Prostanoids in health and disease. J Lipid Res 50 Suppl: S423-S428.
- Haeggestrom JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A (2010) Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun 396: 135-139.
- Morisseau C, Hammock BD (2013) Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 53: 37-58.
- Deng Y, Theken KN, Lee CR (2010) Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol 48: 331-341.
- Xu X, Zhang XA, Wang DW (2011) The roles of CYP450 epoxigenases and metabolites, epoxyeicosatrienioc acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev 63: 597-609.
- Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E (2008) Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 27: 284-330.
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, et al. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276-1279.
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, et al. (2013) Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS One 8: e80922.
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, et al. (2006) Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci 79: 2311-2319.
- Zhao C, Shichi H (1995) Immunocytochemical study of cytochrome P450 (1A1/1A2) induction in murine ocular tissues. Exp Eye Res 60: 143-152.
- Doshi M, Marcus C, Bejjani BA, Edward DP (2006) Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp Eye Res 82: 24-32.
- Bejjani BA, Xu L, Armstrong D, Lupski JR, Reneker LW (2002) Expression patterns of cytochrome P4501B1 (cyp1b1) in FVB/N mouse eyes. Exp Eye Res 75: 249-257.
- Zhao C, Schwartzman ML, Shichi H (1996) Immunocytochemical study of cytochrome P450 4A induction in mouse eye. Exp Eye Res 63: 747-751.
- Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, et al. (1998) Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P450B1. Am J Hum Genet 62: 573-584.
- Stoilov I, Rezaie T, Jansson I, Schenkman JB, Sarfarazi M (2004) Expression of cytochrome P4501b1 (Cyp 1b1) during early murine development. Mol Vis 30: 629-636.
- Vafeas C, Mieyal PA, Urbano F, Falck JR, Chauhan K, et al. (1998) Hypoxia stimulates the synthesis of cytochrome P450-derived inflammatory eidosanoids in rabbit corneal epithelium. J Pharmacol Exp Ther 287: 903-910.
- Mastyugin V, Aversa E, Bonazzi A, Vafaes C, Mieyal P, et al. (1999) Hypoxia-induced production of 12-hydroxyeicosanoids in the corneal epithelium: Involvement of a cytochrome P4504B1 isoform. J Pharmacol Exp Ther 289: 1611-1619.
- Mieyal PA, Bonazzi A, Jiang H, Dunn MW, Schwartzman ML (2000) The effect of hypoxia on endogenous corneal epithelial eicosanoids. Invest Ophthalmol Vis Sci 41: 2170-2176.
- Ashkar S, Mesentsev A, Zhang WX, Mastyugin V, Dunn MW, et al. (2004) Retinoic acid induces corneal epithelial CYP4B1 gene expression and stimulates the synthesis of inflammatory 12-hydroxyeicosanoids. J Ocular Pharmacol Ther 20: 65-74.
- Birkle DL, Sanitato JJ, Kaufman HE, Bazan NG (1986) Arachidonic acid metabolism to eicosanoids in herpes virus-infected rabbit cornea. Invest Ophthalmol Vis Sci 27: 1443-1446.
- Vasiliou V, Gonzalez FJ (2008) Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol 48: 333-358.
- Conners MS, Stoltz RA, Davis KL, Dunn MW, Abraham NG, et al. (1995) A closed eye contact lens model of corneal inflammation. Part 2: Inhibition of cytochrome P450 arachidonic acid metabolism alleviates inflammatory sequelae. Invest Ophthalmol Vis Sci 36: 841-850.
- Seta F, Patil K, Bellner L, Mezentsev A, Kemp R, et al. (2007) Inhibition of VEGF expression and corneal neovascularization by siRNA targeting of cytochrome P450 4B1. Prostaglandins Other Lipid Mediat 84: 116-127.
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD (2007) The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol 50: 225-237.
- Shen HC, Hammock BD (2012) Discovery of inhibitors of soluble epoxide hydrolase: A target with multiple potential therapeutic indications. J Med Chem 55: 1789-1808.
- Kim IH, Nishi K, Kasagami T, Morisseau C, Liu JY, et al. (2012) Biologically active ester derivatives as potent inhibitors of the soluble epoxide hydrolase. Bioorg Med Chem Lett 22: 5889-5892.
- Wagner K, Inceoglu B, Dong H, Yang J, Hwang SH, et al. (2013) Comparative efficacy of 3 soluble epoxide hydrolase inhibitors in rat neuropathic and inflammatory pain models. Eur J Pharmacol 700: 93-101.
- Revermann M (2010) Pharmacological inhibition of the soluble epoxide hydrolase-from mouse to man. Curr Opin Pharmacol 10: 173-178.
- Spector AA, Kim HY (2015) Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta 1851: 356-365.
- Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD (2001) Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 25: 434-438.
- Hwang SH, Tsai HJ, Liu JY, Morrisseau C, Hammock BD (2007) Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem 50: 3825-3840.
- Guedes AG, Morisseau C, Sole A, Soares JH, Ulu A, et al. (2013) Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis. Vet Anaesth Analg 40: 440-448.
- Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, et al. (2001) Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 80: 263-270.
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A (1996) Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 80: 332-336.
- de Smet MD, Taylor SR, Bodaghi B, Miserocchi E, Murray PI, et al. (2011) Understanding uveitis: the impact of research on visual outcomes. Prog Ret Eye Res 30: 452-470.
- Gupta SK, Agarwal R, Srivastava S, Agarwal P, Agrawal SS, et al. (2008) The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Invest Ophthalmol Vis Sci 49: 4036-4040.
- Nussenblatt RB, Calogero D, Buchen SY, Leder HA, Goodkin M, et al. (2012) Rabbit intraocular reactivity to endotoxin measured by slit-lamp biomicroscopy and laser flare photometry. Ophthalmology 119: e19-e23.
- Ghosn CR, Li Y, Orilla WC, Lin T, Wheeler L, et al. (2011) Treatment of experimental anterior and intermediate uveitis by a dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci 52: 2917-2923.
- Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120: 3073-3083.
- Horai R, Caspi RR (2011) Cytokines in autoimmune uveitis. J Interferon Cytokine Res 31: 733-744.
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, et al. (2006) Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A 103: 13646-13651.
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, et al. (2005 ) Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 102: 9772-9777.
- Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, et al. (2010) Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci 40: 222-238.
- Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, et al. (2010) 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem 53: 7067-7075.
- Morisseau C, Hammock BD (2007) Measurement of soluble epoxide hydrolase activity in techniques for analysis of chemical biotransformation. In: Bus JS, Costa LG, Hodgson E, Lawrence DA, Reed DJ (Eds). Current protocols in toxicology, John Wiley & Sons, Hoboken, NJ, 4.23, pp. 1-18.
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, et al. (1999) Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci U S A 96: 8849-8854.
- Diaz-Llopis M, Garcia-Delpech S, Salom D, Udaondo P, Bosch-Morell F, et al. (2007) High-dose infliximab prophylaxis in endotoxin-induced uveitis. J Ocular Pharmacol Ther 23: 343-350.
- Samudre SS, Lattanzio FA Jr, Williams PB, Sheppard JD Jr (2004) Comparison of topical steroids for acute anterior uveitis. J Ocular Pharmacol Ther 20: 533-547.
- Buchen SY, Calogero D, Hilmantel G, Eydelman MB (2012) Detecting endotoxin contamination of ophthalmic viscosurgical devices: Intracameral versus intravitreal assays in rabbits. Ophthalmology 119: e11-e18.
- Buchen SY, Calogero D, Hilmantel G, Eydelman MB (2012) Rabbit ocular reactivity to bacterial endotoxin contained in aqueous solution and ophthalmic viscosurgical devices. Ophthalmology 119: e4-e10.
- Hackett RB, McDonald TO (1996) Ophthalmic toxicology and assessing ocular irritation. In: Marzulli FN, Maibach HI, (Eds). Dermatotoxicology, (4th edn). Hemisphere Publishing Corporation, Washington DC, pp. 749-815.
- Martin PL, Miller PE, Mata M, Christian BJ (2009) Ocular inflammation in cynomolgus macaques following intravenous administration of a human monoclonal antibody. Int J Toxicol 28: 5-16.
- Munger RJ (2002) Veterinary ophthalmology in laboratory animal studies. Vet Ophthalmol 5: 167-175.
- Liu JY, Lin YP, Qui H, Morisseau C, Rose TE, et al. (2013) Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci 48: 619-627.
- Sirish P, Li N, Liu JY, Lee KS, Hwang SH, et al. (2013) Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci U S A 110: 5618-5623.
- Tugal-Tutkun I, Herbort CP (2010) Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol 30: 453-464.
- Fife KL, Liu Y, Schmelzer KR, Tsai HJ, Kim IH, et al. (2008) Inhibition of soluble epoxide hydrolase does not protect against endotoxin-mediated hepatic inflammation. J Pharmacol Exp Ther 327: 707-715.
- Kundu S, Roome T, Bhattacharjee A, Carnevale KA, Yakubenko VP (2013) Metabolic products of soluble epoxide hydrolase are essential for monocyte chemotaxis to MCP-1 in vitro and in vivo. J Lipid Res 53: 436-446.
- Liu Y, Yin H, Zhao M, Lu Q (2014) TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol 47: 136-147.