Journal of Geriatrics and Palliative Care
Download PDF
Research Article
*Address for Correspondence: Corinna E. Lathan, AnthroTronix, 8737 Colesville Road, Suite L-203, Silver Spring, Maryland, USA, Tel: 301-495-0770; Fax: 301-585-9075; E-mail: clathan@atinc.com
Figure 1 shows results for the four in-clinic tasks that were reliably completed. Two-sample Welch t-tests were used to assess differences between caregivers and patients for these subtests: SRT1 mean difference: 29.67 min-1, t(6.52) = -3.66, 95% CI: -49.18, -10.22;PRT mean difference: 9.02 min-1, t(9.99) = -1.58, 95% CI: -21.71, 3.68; GNG mean difference: 16.14 min-1, t(8.72) = -1.81, 95% CI: -36.47, 4.19; SRT2 mean difference: 42.25 min-1, t(11.91) = -5.66, 95% CI: -58.51, -25.98. Notice that group differences for the SRT1 and SRT2 subtests are significant at the 0.05 level.
The in-home phase of the study consisted of the SRT (single administration), PRT and GNG subtests. For these tests, both patients and caregiver performed similarly to in-clinic (Figure 2). Figure 3shows the DANA administrations taken over the course of the inhome study by dyad and caregiver/patient group. Given the repeated measures aspect of the in-home administrations (i.e., multiple administrations nested under subject), multilevel regression models with intercepts estimated for each subject ID were used to evaluate the effect of Alzheimer’s disease on throughput. The estimated effect was negative for all subtests (PRT: b = -12.80, 95% CI: -23.02, -2.57; GNG: b = -15.76, 95% CI: -29.35, -2.18; SRT: b = -16.22, 95% CI: -39.60, 6.93). Note that for the in-home phase, SRT was the only ubtest not to reach significance at the 0.05 level.
An important element of our findings relates to identification of strategies that simultaneously enhance both patient- and caregivercentered support among people whose lives are affected by AD. For caregivers, one aspect of these strategies involves providing self-caretools that help them assess cognitive performance in a manner that optimizes their ability to care for themselves and the people who epend on them [23]. Availability of these caregiver-centered tools is tied to the economic value of informal caregiving. A recent report indicated that informal dementia caregiving is valued at $218 billion annually [7]. Because there is no resource available to cover the cost of replacing informal dementia care with paid support, efforts to ensure caregivers’ well-being including their ability to care for people with AD and to care for themselves have clear economic and policy implications for countries whose populations continue to age without any obvious service streams to support these demographic changes.
A Pilot to Investigate the Feasibility of Mobile Cognitive Assessment of elderly patients and caregivers in the home
Corinna E. Lathan1* Ian Coffman1, Rita Shewbridge 1, Marissa Lee 1, Rosanna Cirio2, Pasquale Fonzetti2, P. Murali Doraiswamy3, Helaine E. Resnick1
- 1AnthroTronix, 8737 Colesville Road, Suite L-203, Silver Spring, Maryland, USA
- 2Burke Rehabilitation Hospital, White Plains, New York, USA
- 3Duke Institute for Brain Sciences, Durham, North Carolina, USA
*Address for Correspondence: Corinna E. Lathan, AnthroTronix, 8737 Colesville Road, Suite L-203, Silver Spring, Maryland, USA, Tel: 301-495-0770; Fax: 301-585-9075; E-mail: clathan@atinc.com
Citation: Lathan CE, Coffman I, Shewbridge R, Lee M, Cirio R, et al. A Pilot to Investigate the Feasibility of Mobile Cognitive Assessment of elderlypatients and caregivers in the home. J Geriatrics Palliative Care 2016;4(1): 6.
Copyright © 2016 Lathan CE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Geriatrics and Palliative Care | ISSN: 2373-1133 | Volume: 4, Issue: 1
Submission: 16 May, 2016 | Accepted: 07 June, 2016 | Published: 11 June, 2016
Submission: 16 May, 2016 | Accepted: 07 June, 2016 | Published: 11 June, 2016
Abstract
Background:The number of older adults with Alzheimer’s disease (AD) has been steadily increasing and is likely to triple by 2050. Parallel increases in AD and informal AD caregivers who experience their own physical and cognitive challenges will result in the need for tools that can help both populations track their cognitive health easily, both in the clinic and at home.Methods: DANA, a tablet-based, FDA-cleared computerized cognitive assessment tool, was used over 90 days among seven caregiver-AD patient dyads in-clinic and at home to assess DANA’s sensitivity in detecting mild cognitive impairment and dementia as wellas its feasibility in the home and clinic.
Results:DANA is sensitive to certain differences in cognitive performance between AD patients and caregiver. Most subtests were found to be feasible for in-home use among both patients and caregivers.
Conclusion:DANA shows promise for use both in-clinic and in the home to track cognitive performance of AD patients and their caregivers.
Keywords
Cognitive performance; Caregivers; Mild Alzheimer’s Disease; DementiaIntroduction
About one in nine Americans aged 65 and older has Alzheimer’s disease (AD), a proportion that increases to one in three among people 85 and older [1]. The aging of the U.S. population, as well asthose in other industrialized countries, has resulted in marked growth in the numbers of older adults who live long enough to experience the debilitating impact of AD [2,3]. In addition to their increasing numbers, these older adults are also growing as a proportion of the total population. In the United States in 1900, there were about 3.1 million adults over the age of 65, and these individuals accounted for 4.1% of the total population. By contrast, in 2050, it is estimated that there will be 88.5 million adults over the age of 65, and this group will represent 20.2% of the population [4]. Assuming no new medical breakthroughs, it has been estimated that the number of AD cases will triple by 2050 from about 5 million to an estimated 13.8 million [5].Advocacy organizations and policy makers have focused heavily on the need to develop effective treatments, service streams, and supports for AD. Passage of the 2011 National Alzheimer’s Project Act (NAPA) [6] called for coordinated efforts to accelerate AD research, provide better care, and improve services for patients andfamilies. NAPA also established an Advisory Council for Alzheimer’s Research, Care, and Services. This group formulated a plan to address AD, including a clear set of objectives aimed at finding effective interventions and treatments [7].
Among the objectives outlined by the NAPA Advisory Council are several that focus on caregivers. The vast majority of day-to-day care for people with AD is provided by informal caregivers, and the extent of this care is considerable. In 2013, Americans provided 17.7 billion hours of unpaid care to people with AD and other dementias, [8] and in 2014, more than 15 million family members and other unpaidcaregivers provided care to these individuals [9]. This translates to 21.9 hours of care per caregiver each week, or 1,139 hours of care per caregiver each year. It is well-established that the vast majority of Alzheimer’s care is provided in the home by unpaid caregivers.
The important role that is played by informal AD caregivers has generated growing interest in the characteristics and well-being of this population. National survey data show that 60% of caregivers of people with AD or dementia are adult children of the care recipient, 21% are over the age of 65, 51% are caring for someone over the age of 85, 23% have cared for the recipient for more than 5 years, 26% reported they have a disability, and nearly 17% report providing more than 40 hours of care each week. Ninety-four percent of AD caregivers in this survey reported that their care recipient experienced a change in thinking or memory in the past year [10].
Given the extent of their caregiving responsibilities, it is perhaps not surprising that AD caregivers are at increased risk of impaired cognition, depression, anxiety, and absenteeism, that they usehealthcare services at higher rates than non-caregivers, and that their mental and physical health decreases as the severity of the AD care recipient’s symptoms increases [11,12]. Numerous reports have shown that caregiving itself is associated with unfavorable effects on various aspects of cognitive function due to factors such as stress and depression [13-20].
The availability of convenient tools to assess cognitive performance is therefore applicable not only to AD patients but also to the caregivers themselves as a means to monitor their own cognitive trajectories. Ideally, such a tool would be easy to use,acceptable to both patients and caregivers, suitable for use in the home, and would provide real-time, actionable information that is useful to the caregiver for both caregiving and self-care. If successfully mplemented, such a tool could help caregivers (1) by providing them with objective information to track the cognitive trajectories of AD care recipients, and (2) by providing information on their own cognitive performance so that they can better understand andrespond appropriately to the challenges imposed by their caregiving role. However, translation of cognitive assessment tools from clinicbased to in-home use among Alzheimer’s disease-caregiver dyads needs to be demonstrated not only to ensure that appropriate tests are selected for home use but also to show that these tests are sensitive to cognitive deficits as measured in the home, as this is where most caregiving occurs.
With these considerations in mind, the objectives of this report areto: (1) assess the in-clinic feasibility of administering a battery of tests via a mobile cognitive performance instrument among Alzheimer’s disease-caregiver dyads; (2) assess the sensitivity of this instrument for detecting mild cognitive impairment (MCI) and dementia and (3)test the feasibility of this instrument for assessing in-home cognitive performance.
Materials and Methods
ParticipantsAD patient-caregiver dyads were recruited at the Burke Rehabilitation Hospital in White Plains, New York. The Burke Rehabilitation Hospital is an acute rehabilitation hospital thatprovides inpatient and outpatient care services. AD Patients were recruited from the outpatient Memory Evaluation and Treatment Service (METS) program, where patients are assessed and treated for memory disorders. Participants included patients diagnosed with mild Alzheimer’s disease and their informal caregivers. The study was approved by the Institutional Review Board of Burke Rehabilitation Hospital.
Inclusion criteria for the dyads included minimum education and age requirements, a Geriatric Depression Scale score of less than six, and English language fluency. Caregiver-specific inclusion criteriaalso required no abnormal memory complaints, scores within normal range on the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) and no clinical diagnosis of dementia, mild cognitive impairment or Alzheimer’s disease. Patient-specific inclusion criteria also required either no medications or stable history of medication usage for three months, meeting NINCDS/ADRDA criteria for probable Alzheimer’s disease and an MMSE score of greater than or equal to 20.
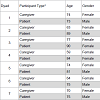
Prior to testing, patients and caregivers were screened by the site PI. Mild AD patients were established patients with previous diagnoses. The PI verified diagnoses and no new diagnostic screenings were conducted for the study. Caregivers were givenstandard neuropsychological tests (i.e. MMSE, MoCA). The site PI performed a history and neuropsychiatric exam to verify eligibility. emographic information for the dyads is shown in Table 2.
Testing
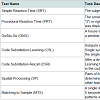
All participants were administered DANA, a tablet-based, FDAcleared neurocognitive assessment tool. DANA contains a battery of tests that is designed to examine cognitive performance on a number of distinct tasks, and its favorable psychometric properties and testretest reliability have been documented [21,22]. A summary of thetests used in this study is provided in Tables 2.
The primary outcome variable for each test is throughput (TP), a measure of cognitive efficiency.
Throughput relates speed and accuracy by quantifying the number of correct responses per minute:
TP = accuracy × speed × 60,000
where accuracy is the proportion of correctly completed trials, speed is the reciprocal of mean correct response time measured in milliseconds. The scaling factor of 60,000 converts the quantity to units of min-1.
If a participant scored less than 66% correct on any test in the battery, results of that test were considered invalid and excluded from analysis. In the context of this study, such performance is indicative of the inability to perform a particular task.
Testing was carried out in two settings: a clinic-based setting at the Burke Rehabilitation Hospital and in patients’ homes. The firsttesting session took place in clinic, where both caregivers and patients were administered the complete range of tests described in Tables 1. For in-home testing, each patient-caregiver dyad was provided with a tablet running DANA software and instructed to complete the assessment at home at least once a week for 90 days. For in-home testing, a complete administration consisted of the Simple Reaction Time, Procedural Reaction Time, and Go/No-Go subtests.
At the end of the 90-day home testing portion of the study, caregivers were contacted to take a follow-up survey soliciting feedback regarding their experience with DANA.
Results
In-clinic test administrations are shown in Table 2. AD patients were unable to reliably omplete many of the tests that have been used previously in the DANA cognitive test battery, ncluding CSL (1/7 patients completed), CSR (0/7), SP (3/7), and MTS (3/7). However, the patients ad greater success in completing the simpler processing speed tasks: SRT1 (7/7), PRT (5/7), GNG 6/7), and SRT2 (7/7). As indicated, caregivers were also unable to complete many of the assessments.1The Simple Reaction Time subtest was administered twice: once at the beginning of the battery and once at the end. These two administrations are labeled as SRT1 and SRT2, respectively, in what follows.
Post-study follow-up interviews indicated that a majority of caregivers were able to independently set up the tablet and support the patient during the data collection period. Caregivers provided feedback on the device being used (a tablet) and the perceived usefulness of the in-home cognitive assessment. Caregivers providedadditional feedback on DANA regarding instructions, stimulus size, and software navigation. Additionally, they reported generally positive impressions concerning perceived benefits of taking the assessment at home for both themselves and the patient.
Discussion
This study had three goals: (1) to assess the in-clinic feasibility of administering a battery of tests via a mobile cognitive performance instrument among Alzheimer’s disease-caregiver dyads, (2) to assess the sensitivity of this instrument for detecting mild cognitiveimpairment (MCI) and dementia, and (3) to test the feasibility of this instrument for assessing in-home cognitive performance. Each is discussed below.We found that DANA’s full cognitive battery was not appropriate for our sample, particularly among AD patients. Patients were unable to reliably complete certain tasks such as Code Substitution, Matching to Sample, and Spatial Processing. Caregivers also had difficulty withsome tasks, perhaps as a consequence of their advanced age. These tasks could potentially be modified for clinic use (such as increasing available response time). By contrast, simpler tasks like Simple Reaction Time, Procedural (Choice) Reaction Time, and Go/No-Go were generally reliably completed by both groups.
Despite our small sample size, in-clinic testing revealed numerical trends consistent with the expected result that the Alzheimer’s group would perform worse than caregivers across a range of cognitive tests. These trends were also observed in the home, suggesting a reliable transfer of DANA’s sensitivity to Alzheimer’s disease that was demonstrated in the clinical setting. Although in some casesdifferences in cognitive performance between caregiver and patient groups failed to reach traditional significance thresholds, we believe that more consistent results will be obtained with larger sample sizes and/or through measurement of factors that are likely to contribute to variance in cognitive performance (e.g., medication, stress, etc.).
Finally, our results speak to the feasibility of using a portable neurocognitive assessment tool in the home. Although the number of administrations varied among participants, testing sessions spanned the entire range of the study period and were generallyevenly distributed across it (Figure 3), suggesting that engagement with the device was consistent over the course of the study. Results of the follow-up questionnaire provided useful insights into the usability concerns among caregivers and patients, thereby providing a platform for further development of this testing modality in this population.
An important element of our findings relates to identification of strategies that simultaneously enhance both patient- and caregivercentered support among people whose lives are affected by AD. For caregivers, one aspect of these strategies involves providing self-caretools that help them assess cognitive performance in a manner that optimizes their ability to care for themselves and the people who epend on them [23]. Availability of these caregiver-centered tools is tied to the economic value of informal caregiving. A recent report indicated that informal dementia caregiving is valued at $218 billion annually [7]. Because there is no resource available to cover the cost of replacing informal dementia care with paid support, efforts to ensure caregivers’ well-being including their ability to care for people with AD and to care for themselves have clear economic and policy implications for countries whose populations continue to age without any obvious service streams to support these demographic changes.
Our findings can also be interpreted in the context of NAPA’s plan to address AD. Goal 3 of that plan is to “Expand support for people with Alzheimer’s disease and their families”. Strategy 3B ofthat plan calls for enabling “family caregivers to continue to provide care while maintaining their own health and well-being” and strategy 3C seeks to “Assist families in planning for future care needs” [24]. Given that about half of all AD caregivers are themselves over the age of 55 - a finding that is reflected in our data - the needs of these aging caregivers must be addressed in parallel with the needs of their care recipients. Our data on the feasibility of using a homebased cognitive assessment tool is consistent with federal priorities to support caregivers as well as AD patients with tools that support their needs, help maintain caregiver health, and assist in planning for future care needs.
It is the context of supporting caregivers in this important role that our findings are especially relevant in a public policy context. The average per-person Medicare spending for seniors with Alzheimer’sis almost three times higher than average per-person spending for all other seniors. Under Medicaid, spending is 19 times higher [24]. It is important to stress that these costs are associated with formal health care provision, and that effective provision of informal care helps to keep these costs down.
Although the aging of the population will undoubtedly result in increasing numbers of older adults who will continue to incur substantial costs to the formal health care system, it may be possibleto control these costs by offering caregivers effective tools that can optimize their ability to provide informal care.
Acknowledgements
This study was funded by the BrightFocus® Foundation and the Geoffrey Beene Foundation Alzheimer’s Initiative. Disclosure Statement: The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. AnthroTronix is the developer of the DANA. Dr. P. Murali Doraiswamy has received grants, advisory or speaking fees and/or stock from several companies. He is an advisor and minor shareholder in AnthroTronix.References
- Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer’s disease in the United States (2010-2050) estimated using the 2010 Census. Neurology 80: 1778-1783.
- Ortman JM, Velkoff VA, Hogan H (2014) An aging nation: the older population in the United States: Current population reports. US Census Bureau, Washington, DC, pp. 25-1140.
- Eurostat Statistics Explained (2016) Population structure and ageing.
- ACL (2016) Administration on Aging (AoA) projected future growth of the older population.
- Lathan C, Spira JL, Bleiberg J, Vice J, Tsao JW (2013) Defense Automated Neurobehavioral Assessment (DANA) - psychometric properties of a new field-deployable neurocognitive assessment tool. Mil Med 178: 365-371.
- Authenticated US Government Information (2011) National alzheimer’s project act. pp. 111-375.
- NASPE (2015) National plan to address alzheimer's disease: 2015 update. pp. 1-58.
- Alzheimer’s Association (2014) 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10: e47-e92.
- Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11: 332-384.
- Caregiving Across the United States (2009) Caregivers of persons with alzheimer’s disease or dementia in 8 states and the district of Columbia.
- Kannan H, Bolge SC, Del Valle M, Alvir J, Petrie CD (2011) The association between Alzheimer's disease symptom severity and caregiver outcomes: a cross-sectional study. Prim Care Companion CNS Disord 13.
- Pinquart M, Sorensen S (2003) Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging 18: 250-267.
- Lathan C, Wallace AS, Shewbridge R, Ng N, Morrison G, et al. (2016) Cognitive health assessment and establishment of a virtual cohort of dementia caregivers. Dement Geriatr Cogn Dis Extra 6: 98-107.
- Mackenzie CS, Smith MC, Hasher L, Leach L, Behl P (2007) Cognitive functioning under stress: evidence from informal caregivers of palliative patients. J Palliat Med 10: 749-758.
- Caswell LW, Vitaliano PP, Croyle KL, Scanlan JM, Zhang J, et al. (2003) Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Exp Aging Res 29: 303-318.
- Lee S, Kawachi I, Grodstein F (2004) Does caregiving stress affect cognitive function in older women? J Nerv Ment Dis 192: 51-57.
- Oken BS, Fonareva I, Wahbeh H (2011) Stress-related cognitive dysfunction in dementia caregivers. J Geriatr Psychiatry Neurol 24: 191-198.
- Gaugler JE, Davey A, Pearlin LI, Zarit SH (2000) Modeling caregiver adaptation over time: the longitudinal impact of behavior problems. Psychol Aging 15: 437-450.
- Vitaliano PP, Zhang J, Young HM, Caswell LW, Scanlan JM et al. (2009) Depressed mood mediates decline in cognitive processing speed in caregivers. Gerontologist 49: 12-22.
- Corrêa MS, Vedovelli K, Giacobbo BL, de Souza CE , Ferrari P, et al. (2015) Psychophysiological correlates of cognitive deficits in family caregivers of patients with Alzheimer Disease. Neuroscience 286: 371-382.
- Russo CR, Lathan CE (2015) An evaluation of the consistency and reliability of the defense automated neurocognitive assessment tool. Appl Psychol Meas 39: 566-572.
- Miller LS, Lewis MS, Williamson GM, Lance CE, Dooley WK, et al. (2006) Caregiver cognitive status and potentially harmful caregiver behavior. Aging Ment Health 10: 125-133.
- Chari AV, Engberg J, Ray KN, Mehrotra A (2015) The opportunity costs of informal elder-care in the United States: new estimates from the American Time Use Survey. Health Serv Res 50: 871-882.
- Alzheimer’s Association (2016) Costs of alzheimer’s to medicare and medicaid.