Journal of Food Processing & Beverages
Download PDF
Review Article
*Address for Correspondence: Aman Ullah, 4-10 Agriculture/Forestry Centre, Department of Agricultural, Food and Nutritional Science, University of Alberta, T6G 2P5, Tel: (780)-492-4845; Fax: (780)-492-4265; E-mail: amanullah@ualberta.ca
Citation: Khosa MA, Ullah A. A Sustainable Role of Keratin Biopolymer in Green Chemistry: A Review. J Food Processing & Beverages. 2013;1(1): 8.
Copyright © 2013 Aman Ullah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 1
Submission: 15 August 2013 | Accepted: 14 September 2013 | Published: 22 September 2013
A Sustainable Role of Keratin Biopolymer in Green Chemistry: A Review
Muhammad A Khosa and Aman Ullah*
- Department of Agriculture, Food & Nutritional Science, University of Alberta, Edmonton, AB
*Address for Correspondence: Aman Ullah, 4-10 Agriculture/Forestry Centre, Department of Agricultural, Food and Nutritional Science, University of Alberta, T6G 2P5, Tel: (780)-492-4845; Fax: (780)-492-4265; E-mail: amanullah@ualberta.ca
Citation: Khosa MA, Ullah A. A Sustainable Role of Keratin Biopolymer in Green Chemistry: A Review. J Food Processing & Beverages. 2013;1(1): 8.
Copyright © 2013 Aman Ullah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 1
Submission: 15 August 2013 | Accepted: 14 September 2013 | Published: 22 September 2013
Abstract
A great deal of environmental concerns, oil price hike, and rapid oil consumption with finite nature of oil reserves in addition to consumer demand is driving research into renewable, biodegradable, inexpensive, and abundantly available biopolymers in material chemistry. Poultry feathers consist of keratin protein with several amino acids especially cysteine as a major component. Keratin is an original raw material that has great potential to be used in the development of novel fibrous composite materials. Being very effective biopolymer, keratin possesses numerous functional properties, bioactivities, and chemical applications in material chemistry, for example, keratin based gels, films, nano/micro-particles, and beads are of paramount importance. Modified keratin also serves as a biosorbent for removing toxic metal ions from water resources on account of exceptionally important role of functional groups for keratin-metal binding. This review focuses on the recent advances of keratin in material chemistry including its significant role as biosorbent in green chemistry.Introduction
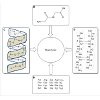
Green chemistry has many challenges; for example, improvement in research, development, and implementation of innovative chemical technologies that accomplish pollution prevention in a scientifically sound and cost-effective manner. To accomplish these objectives, research in green chemistry recognizes and supports chemical technologies that reduce or eliminate the use or generation of hazardous substances during the design, manufacture, and use of chemical products and processes. Targets in the development of green materials include bioplastics, films, packaging, building materials, and fibers [1-3]. Bio-based renewable materials are considered safer than synthetic fossil fuel-derived materials. Research on several proteins, including collagen, gelatin, albumin, fibroin and keratin is in progress for the development of naturally-derived biomaterials. For instance, Poole et al. concluded that sustainable fiber obtained from regenerated protein was environmental friendly, renewable and biodegradable. Similarly, Xiao-Chun et al. extracted keratin effectively from chicken feather and fabricated keratin films which were used in controlled drug delivery systems [4,5]. Among these proteins, development of keratin-based material has the potential for revolutionizing the bio-based green materials’ world due to their biodegradability, biocompatibility, mechanical durability, and natural abundance [4,6]. Over the past century, research has focused on the extraction, purification, characterization, and applications of keratin protein from hair, nails, and wool fibers [7]. Chicken feathers (CF) are perhaps the most abundant keratinous material in nature [8]. Chicken meat produces feathers approximately 5 million tonnes as a waste stream per year and meat processing occurs throughout the year particularly, in centralized locations and the collected feathers have minimal value in poultry industry. Apart from its minor consumption in low grade animal feed, disposal of remaining bulk poses a significant environmental threat to poultry farming industry in addition to landfill, thus making transport the main cost of the raw material. Therefore, from economic and environmental point of views, it is quite desirable to mechanize lucrative and effective process to use these kind of sources in green chemistry [9]. Feather keratins are small proteins, uniform in size, with a molecular weight around 10 kDa [10,11]. They constitute cysteine, hydrophobic residues and β-sheet conformations [8,12,13]. Several functional groups present in keratin protein, especially peptide backbone, such as disulfide (-S-S), amino (-NH2) and carboxylic acid (-COOH), make it chemically reactive under conducive reaction conditions. Apparently, keratin is insoluble in water with very low chemical reactivity. However, its solubility in water increases at low/acidic pH, high temperature and in presence of some reducing agent (i.e. Na2SO3 or Na2S). On reduction, disulfide cross-links are broken into free thiol (-SH) besides protonation of some -NH2 and other groups in keratin making its surface positive and thus solubilisation takes place. After protonation, unfolded and exposed functional groups carry positive surface charges with high reactivity and therefore, on chemical modification, keratinous protein becomes pseudo natural cationic biopolymer. With unique properties of bio-degradability and non-toxic nature, keratin protein is versatile biopolymer that can be modified and developed in various forms, for instance, gels, films, beads and nano/micro-particles. After modification, it finds numerous applications in green chemistry, food sciences, pharmaceutical, and cosmetic industries. Different components of keratin protein have been shown in Figure 1.Extraction of Keratin
The word keratin first appeared in the literature around 1850 to describe the material which is made up of hard tissues such as animal hoofs and horns. Keratin comes from the Greek word “kera” which means horn. Over the years, keratin has been extracted from different sources such as hair, horn, hooves, beaks, shells, fingernails, toenails, claws and feathers. A Chinese herbalist Shi-Zhen Li used keratin in medicine for the first time in 16th century. In 1905, a patent was issued by John Hoffmeier from United States describing the process of keratin extraction from animal hoofs with help of lime [7]. The extracted keratin was further employed for making gels using formaldehyde as hardening agent. Many methods were developed to extract keratin using oxidative and reductive chemistry [14-19]. These technologies were initially applied on animals’ horns, hoofs, thereafter, on chicken feathers and finally human hair for extracting keratin. Mainly keratin was extracted by many researchers from poultry feathers under reducing conditions using Shindai method [20-25]. The extraction process consists of three steps: ethanol pretreatment, hydrochloric acid pre-treatment and 2-mercaptoethanol deoxidization. All reactions are performed in a flask containing 150-200 ml reduced keratin solution and researchers use 5ml of chloroacetic acid solution (0.15g mL-1) drop wise while regulating pH value to 8-9 and reaction is stopped after one hour. Keratin solution is acidified and precipitated with ethanol and keratin sedimentation is washed for three to four times before it is lyophilized [5]. Advances in keratin extraction, purification and characterization led to the potential growth in keratin-based green material development. Keratin after extraction, was potentially used to prepare powders, films, gels, coatings, fibers, and foams by many researchers such as Krystayna et al. [26] prepared fibrous composites; Ankar CA [27] prepared keratin containing films and coating material; Kawano YO, S [28] sythesized keratin based film and gelatin; and Noishiki Yi [29] applied denatured wool keratin derivatives to an dntithrombogenic biomaterial-vascular graft coated with a hyperinized keratin derivatives. The chemical properties of keratin are both weak acids and bases. Keratin is characterized by cystine content in amino acids sequence and it can be reduced, oxidized and hydrolyzed. Its high strength is on account of cysteine molecules bonded by disulfide bonds [30].Keratin Modification
In material chemistry, covalent modification of keratin is an effective way to modulate macromolecular function [31-33] and it is important to comprehend exact role of these alterations. Complicating the analysis of post-translational modifications is the limited provision of pure and naturally modified proteins. Chemically modified proteins resolve issues of homogeneity and availability of material for chemical applications. However, chemical modifications of keratinous protein are selective for molecular residue of protein yielding suitable natural or mimic modified biomaterial [34]. In addition, chemical modification of proteins may be used for unnatural alterations in the reactivity of desired functional groups. The development of selective chemical reactions which yield well-defined keratin macromolecules with tailored properties is pushing research in understanding of biological processes with modified proteins. However, the major challenge is the selective modification of particular residue of keratin in presence of numerous competing side chains of polypeptide. A care must be taken to maintain reaction conditions required for avoiding protein denaturation. Many researchers employed different electrophiles in direct alkylation of cysteine residues [35,36]. In 1935, alkylation of cysteine was accomplished using electrophile such as iodoacetamides for the first time [20]. Chalker et al. [37] carried out chemical modification of cystein molecule using keratinous protein lately in 2009. Whereas, in 1978, Clark and Lowe [38] had already worked on conversion of the active-site cysteine residue of papain into a dehydro-serine, a serine and a glycine Residue. They used bromoacetophenone derivatives to alkylate cysteine of keratin and converted into formyl glycine photochemically. Selective alkylation of keratinous cysteine was carried out by its conjugate addition to Michael acceptors [36,39]. Moore and Ward [40] brought reduction in disulfide bonds and thereafter replaced the crosslink with bismaleimides adduct. Investigators continued using maleimides to the present day for an effective alkylation during modification of keratinous cysteine [41]. The formation of disulfides is dependent on chemical oxidation of cysteine in modification of keratin especially in chemo-selective legation. Many simple and complex methods were adopted to form disulfide on keratinous cysteine; for example, air oxidation, mixing thiol in protein containing basic buffer, and reaction of 5,5-dithiobis (2-nitrobenzoate) or Ellman’s reagent with cysteine [36,42]. Similarly, few researchers used iodine and sulfenyl halides as reductant for mixed disulfide formation apart from activated thiols [43-45]. Wieland and co-workers [46] gave idea of seminal ligations leading to synthesis of new modified protein which works on the basis desulfurization of cysteine after native chemical legation (NCL). Recently, two researchers Danishefsky and Wan [47] have developed a mild radical based desulfurization that was specified for cysteine. Such reactions regarding desulfurization have also been reported by Crich et al. on model peptides [48-51]. Holmes and Lawton [52], for the first time, reported about conversion of cysteine into dehydroalanine as a result of its oxidative elimination during desulfurization. Some metals have also been reported to mediate chemistry of keratinous cysteine due to its natural affinity towards metals [53]. For instance, nickel and palladium are used to mediate the selective reduction of cysteine to alanine [54]. Likewise, masking the reactivity of cysteine until needed was accomplished by using Zncomplexes in modification of keratin [55].Keratin-based Biomaterials
A term “keratin” stands for broad category of insoluble proteins associated as intermediate filaments (IFs) which are responsible for the formation of excessive epithelia and epidermal Appendageal Structures such as hair, wool, horns, hooves and nails. Bonser et al. reported structure and properties of keratin extracted from different sources for instance, feather rachis, nails, hoofs and hair [56]. The aspect of employing keratin as a biomaterial in medical application was quite obvious. The solid foundation for the development of many keratin based biomaterials is based on several key properties of keratins that play good role in physical, chemical and biological behavior of these biomaterials. Unique chemical and biological behavior of keratin apart from enhanced need of renewable natural resource, have been boosting factors for research based on biomaterials over the past three decades. Plenty of work has been done to fabricate and characterize new products based on keratin, for example, films, gels, coating material and biosorbents in green chemistry. In many cases, these novel keratin materials are shown to possess excellent biocompatibility. Additionally, many researchers have discovered methods for modulating the physical and mechanical properties of keratins in order to create biomaterials that have appropriate characteristics for their application of interest. The extracted keratin proteins have ability to self-assemble into complex three dimensional structures. This property is responsible for their development as scaffolds in tissue engineering. Tachibana et al. [57] fabricated wool keratin scaffolds for long term cell cultivation in 2001 and created matrices by lyophilisation of aqueous wool keratin solutions after controlled freezing. This resulted in formation of rigid and heatstable structure with a homogenously porous microarchitecture. The keratins incorporated by RGD and LDV cell adhesion sequences, exhibited good cell compatibility by supporting the attachment and proliferation of fibroblasts over a long-term cultivation up to 23-43 days. In addition, the free cysteine residues within the scaffold were potential modification sites to immobilize bioactive substances [57]. In later work, lysozyme was employed as a model compound and linked to the keratin sponge via disulfide and thio-ether bonds. Disulfidelinked lysozyme was gradually released over the period of 21 days whereas lysozyme linked via thio-ether bonds remained stable for two months. This work demonstrated that the selection of a chemical cross-linker can uniquely determine the stability of an immobilized bioactive substance on keratin sponges [58]. The active free thiol in the keratin sponges can be functionalized in various chemical treatments. For example, iodoacetic acid, 2-bromoethylamine, and iodoacetamide were used to produce carboxyl-, amino-, and amido-sponges, respectively. In these chemically-modified keratin sponges, extracellular matrix proteins were mimicked, and the large presence of active groups within the sponges were allowed for further hybridization with bioactive molecules. This technique was demonstrated by Tachibana et al. [59] in 2005 via hybridization of keratin sponges treated by calcium phosphate. Keratin carboxysponges were functionalized with bone morphogenetic protein-2 (BMP-2), that associated tightly inside keratin [60]. The pore size regulation and porosity of keratin scaffolds was achieved by Katoh et al. using a compression molding-particulate leaching (CM/PL) technique. The regulation of pore diameter and interconnectivity of scaffolds is always desirable to permit adequate cellular infiltration and nutrient delivery in tissue engineering applications. Moreover, CM/PL method was water tolerable and thus, significantly superior to collagen materials that are water soluble without using UV irradiation or cytotoxic chemical cross-linkers [61]. A relationship between mass and physical strength was established in vivo biodegradation of keratin bars by Peplow et al. In series of experiments, rectangular bars of reconstituted keratins were subcutaneously implanted into adult rats followed by monitoring of dry weight and elastic modulus of the explanted bars over an 18-week time period. The dry weight of the bars lowered gradually with a maximum weight degradation of 22% in 18 weeks. The elastic modulus of the keratin bars diminished abruptly between 3 and 6 weeks accompanied by raising number of fissures and cavitations at the surface of the bars. The gradual degradation and rapid loss of mechanical integrity suggests that this form of keratin is more suitable for resorbable implant material to provide scaffolding for non-load bearing applications [62]. Verma et al. recently reported construction, characterization and cyto-compatibility of human hair protein scaffolds for in vitro tissue engineering applications. Keratin proteins from hair were transformed into porous sponges via lyophilization of frozen protein suspension. Characterization results showed that the sponges were capable of swelling 48% within 60 minutes containing average pore diameter of 150 μm in sponge surface. The interconnectivity and pore diameters supported cell attachment and survival and the researchers suggest that these scaffolds are prospective materials for tissue engineering applications on account of their human origin, biodegradability and cytocompatibility [63].Keratin Fibers
The current applications of keratin are in composites and non-woven fabrics [64] and keratin has been characterized for their microstructural properties. Over the years, electrospinning of biocompatible polymeric materials has attracted researchers due to biomedical applications for nanofibrous materials. Electrospinning is a technique that applies high voltage and creates charged jet of polymer drawn towards a grounded collection plate or mandrel. The resulting fibers have very small diameters in the nano- to micro-scale range and are randomly arranged in order to form a non-woven fibrous mat. The enhanced physical configurations such as small pore size, high porosity, three-dimensional features, and high surface area-tovolume ratio of nanostructured nonwoven particulates can promote cell adhesion and growth. This further helped in the development of electrospun membranes, bandages for wound healing and scaffolds for tissue engineering. Recently, the electrospinning process has also been extended to include regenerated keratin extracted from hair and wool fibers. Aluigi et al. [65,66] prepared keratin/PEO materials by mixing aqueous keratin solutions and PEO powder. In the first study, the researchers identified the electrospinning parameters to create defect-free fibrous material. A keratin/ poly (ethylene oxide) (PEO) solution with weight ratio of 50:50 or (7% and 10%) polymer concentrations showed sufficient viscosities for electro spinning with fewer defects. Spectroscopic and thermal data revealed that the electro-spinning process destabilized the natural self-assembly of keratin and caused a less complex protein conformation. Different proportions of keratin and PEO combination were used to correlate the chemical, physical, and rheological properties of the blend solutions with the morphological, structural, thermal and mechanical properties of the electrospun mats. The keratin/PEO solutions were shown to have increased viscosities in comparison to both pure PEO and keratin, and the blends exhibited a non-Newtonian flow behavior with strong shear-thinning properties that were dependent on PEO concentration. The low viscosity of blends with higher keratin content greatly hindered their ability to form fibers; however, solutions with a lower composition of keratin were successfully electro-spun without defects. Comparisons between actual and theoretical rheological properties using Graessley’s theory showed that the broadening of molecular weight distribution and possible bonding between PEO and keratin macromolecules at certain keratin/PEO ratios are responsible for the shear viscosity behavior of the blends, which ultimately correlate with the morphology of the electro-spun fibers. The practical uses of the keratin/PEO nano-fibrous mats, however, were ultimately limited by their water instability and poor mechanical properties [66,67].Keratin Films
Modified films based on keratin extracted from wool and human hair have been used for a number of years to explore the structural and biological properties of self-assembled keratins. Yamauchi et al. [68] investigated the properties of products made from extracted wool keratins and described the physiochemical and biodegradational oroperties of solvent-cast keratin films. Although pure keratin films were too fragile for practical use, but addition of glycerol resulted in a transparent, relatively strong, flexible, and biodegradable film. Fujii et al. also demonstrated usefulness of hair keratin for preparing protein films and described quick casting method. This research also gave the feasibility of incorporating such bioactive molecules as alkaline phosphatase into the keratin films for controlled-release applications. Nonetheless prepared films showed poor strength and flexibility [69] despite the fact these early studies demonstrated feasibility of preparing keratin films and demonstrated their potential use as biomaterials in medical applications. The practical utility of keratin based products was limited to their poor mechanical characteristics. Therefore, keratin film research moved to concentrate on the optimization of the physical strength in addition to flexibility of films by maintaining biological activity. Many approaches to control physical and biological properties have been adopted, for instance, addition of natural and synthetic polymers to keratin blended systems besides the development of new preparation techniques for pure keratin films [70,71]. In 2002, Yamauchi et al. studied the mechanical properties of glycerol containing keratin films by adding chitosan which improved mechanical strength. Furthermore, the chitosankeratin films demonstrated antibacterial properties and proved to be good substrates for cell culture [72]. The biological activity of keratin films was enhanced by incorporating a cell adhesion peptide, Arg-Gly-Asp-Ser (RGDS), at the free cysteine residues of reduced keratin extracts. RGDS-carrying keratin films proved to be excellent substrates for mammalian cell growth, and this work demonstrated the potential and versatility of keratin biomaterials [73]. A natural polymer Silk fibroin (SF) has lately received attention as a biomaterial because of its intrinsic biocompatibility and biodegradability. Keratin-SF films have been studied extensively to understand the interactions between the two biomolecules and its impact on the overall mechanical and biological characteristics of the biomaterial. Lee et al. [74] studied the secondary structure of keratin-SF films and its transition from random coil to β-sheet structure for fibroin due to the presence of the polar amino acids present in keratin. These blended films experimentally enhanced antithrombogenicity properties with high bio-compatibility as compared to mere SF or keratin films [75] mainly on account of enhanced surface polarity of the blends generated by the conformational transformation of the proteins [76]. Vasconcelos et al. explored mechanical and degradation properties of keratin-SF blended films and concluded that SF and keratin interactions are not simply additive. Instead, the two proteins are capable of unique intermolecular interactions directly affecting the bulk properties of the films. Ultimately, the nature, strength of these interactions and knowledge of the degradation rates will help design of matrices for release of active compounds that are suitable for future biomedical applications [77]. Apart from natural biopolymers, the interaction between keratin and synthetic polymers has also been investigated [78,79] and Tonin et al. explored the relationship between poly (ethylene oxide) (PEO) and keratin blended films in order to develop a keratin-based material with improved structural properties. Morphological, structural and thermal analyses of the keratin-PEO films revealed that keratin inhibits PEO crystallization and PEO interferes with the keratin self-assembly at appropriate level by inducing β-sheet secondary protein structure with high thermal stability. The improved structural properties of keratin-PEO blends helps in the development of keratin materials for their possible usage as scaffolds in cell growth wound dressings and drug delivery membranes [78,80]. Researchers have also investigated alternative fabrication techniques for creating keratin films with more suitable mechanical properties in addition to creating blended keratin systems with natural or synthetic polymers. Katoh et al. reported an alternative method for processing keratin films to overcome the limited versatility associated with solution-cast methods. Compression molding of S-sulfo keratin powder gave an effective technique for producing pure keratin films of distinct shape. Control over mechanical properties of the films was obtained by molding temperature and water content of the film. Whereas, the biocompatibility of the S-sulfo films was also evaluated by fibroblast attachment and proliferation on the keratin substrates [71]. The pure keratin films with translucent and flexible properties in a separate study was reported giving improved procedure of its preparation followed by practical application and compatibility of the films was evaluated with human skin [70]. Reichl et al. adopted two different approaches for substrate coatings and studied growth behavior of twelve different cell lines cultured on the keratin films. Results depicted that growth substrates formed by casting of a keratin nano-suspension supported cell adherence and improved cell growth as compared to uncoated polystyrene or keratin coatings formed by trichloroacetic acid precipitation. The new approach is cost effective and alternative to commonly used coatings for example, collagen and fibronectin [81]. Recently, several researchers developed films from native keratin, by using reducing agents and different plasticizers, either via compression molding [82] or extrusion and subsequent compression molding [11,83] leading to improved mechanical properties.Keratin as Biosorbent
Natural biopolymers have certain advantages in green chemistry including their pronounced role in preventing environmental pollution. Keratin application for the purification of metal contaminated natural and waste water resources can be a promising technology. As a matter of fact, researchers started studies on binding of keratin wool to heavy metal ions in the early 1950s. In environmental sciences, removal of heavy metals from drinking and process water is a subject of continued research. The presence of heavy metals in water sources is the result of industrial discharges especially those dealing with mining, manufacturing and processing tasks [84]. Heavy metals pose health hazards to man and aquatic life when exceed allowable limit in water [85]. The concentrations of heavy metals even if below these limits, have potential for long term contamination on account of their accumulative nature in biological systems [86]. There are several water treatment technologies such as precipitation, coagulation/flocculation, cementation, chelation, ion exchange method, micellar and polymer enhanced ultrafiltration [15,16,87-89]. These technologies have limitations of sludge handling or safe disposal besides their suitability only for solution of high metal concentrations. Apart from that, conventional water treatment techniques require complicated operational set up and found to be relatively expensive and selective for few metals. Thus, in recent years, there was increasing interest in the use of biopolymer for the removal of dissolved metals from aqueous solutions [90]. Several metals such as mercury, copper, silver, cadmium, lead, chromium and aluminium were removed using keratin wool by many researchers over the years [91-93]. Another type of keratin namely mohair has been reported to remove copper from aqueous stream [94]. Animal fibrous protein has avian keratin that is extracted from feathers of chicken and turkey and it has been found very effective to segregate metals like copper, removed oxy-anionic contaminants of selenium and arsenic using modified form of keratin from water with high sorption uptake at lower pH value [97]. Gamze-Turan removed Cu(II) and Zn(II) from aqueous stream using poultry litter that is mixture of excreta, feed, bedding material and keratin of chicken feathers [98,99]. In 2009, Sun et al. [100] used keratin of modified chicken feather as biosorbent to remove toxic chromium (VI) ions from water stream. They concluded when chicken feathers are treated with NaOH, the reaction takes place on the surface instead of interior of the feathers. This results in low sorption capacity of keratinous biosorbent for removing Cr(VI) ions in the concentration range of 10-80 ppm [101]. Teixeira MC and Ciminelli VS described a biological route for the sorption of aqueous As(III) species and evaluated that a waste biomass with a high fibrous protein (keratin) content can be used for selective As(III) adsorption [102]. Fawzi Banat et al. examined and compared keratin composed biosorbents prepared from three different sources of chicken feathers, human hair and animal horns for the removal of Zn(II) and Cu(II) ions from water. They found that animal horn based keratin showed greater sorption efficiency than chicken feathers and human hair. The same research group also removed Cu(II) and Zn(II) from wastewater after they reused chicken feathers as biosorbent and treated chemically with NaOH and anionic surfactant dodecyl sulfate [103,104]. The summary of sources, modifications and applications of keratin or keratin derivatives, has been given in Table 1.Future Directions and Novel Applications of Keratin
Although several efforts have been made to date for the development of green materials using extracted or native keratin from different sources, yet there is much left to replace chemically synthesized material by degradable and environmentally friendly biomaterial in industries. For example, as far as biological and chemical behavior of functional groups is concerned, limited efforts have been made on fundamental understanding of poultry feather keratin. Feather keratin is a special protein due to the presence of high amount of cysteine residues inside the polypeptide backbone. These cysteine residues form sulfur-sulfur bonds with other cysteine molecules leading to disulfide bridges (cysteine-cysteine cross-links) which give strength and stiffness to keratin in the solid state. However, these cross-links not only offer hindrance in protein extraction process but also post-extraction efficient modification cannot completely stop oxidation of cysteine residues. Therefore, a pragmatic research is required to be done on to investigate how the cysteinecysteine cross links can be broken after experimental and theoretical identification of scientific gap in such cross links. Development of hybrid nanobiomaterials by in-situ nanomodifications of keratin, through effective exploitation of nanotechnology, can lead to the development of green products with enhanced material properties for applications in textiles, composites, nano-structured biomaterials, and other bioproducts.References
- Hernandez-Munoz P, Kanavouras A, Ng PK, Gavara R (2003) Development and characterization of biodegradable films made from wheat gluten protein fractions. J Agric Food Chem 51: 7647-54.
- Jerrold EW JH, Jessie AM, Ashok R, Walter S (2003) Potential of Chicken Feather Fibre in Wood MDF Composites. EcoComp2003, Queen Marry, University of London.
- Yin J, Rastogi S, Terry AE, Popescu C (2007) Self-organization of Oligopeptides Obtained on Dissolution of Feather Keratins in Superheated Water. Biomacromolecules 8: 800-806.
- Poole AJ, Church JS, Huson MG (2009) Environmentally sustainable fibers from regenerated protein. Biomacromolecules 10: 1-8.
- Yin XC, Li FY, He YF, Wang Y, Wang RM (2013) Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater Sci 1: 528-536.
- Balaji S, Kumar R, Sripriya R, Rao U, Mandal A, et al. (2012) Characterization of keratin–collagen 3D scaffold for biomedical applications. Polym Advan Technol 23: 500-507.
- Jillian GRM, Van Dyke ME (2010) A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 3: 999-1014.
- Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S (1998) A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol 66: 1-11.
- Wang YX, Cao XJ (2012) Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process Biochem 47: 896-899.
- Arai KM, Takahashi R, Yokote Y, Akahane K (1983) Amino acid sequence of feather keratin from fowl. Eur J Biochem 132: 506-507.
- Ullah A, Vasanthan T, Bressler D, Elias AL, Wu J (2011) Bioplastics from Feather Quill. Biomacromolecules 12: 3826-3832.
- Sakurada I, Nukushina Y, Ito T (1962) Experimental determination of the elastic modulus of crystalline regions in oriented polymers. J Polym Sci 57: 651-660.
- LGuhados G, Wan W, Hutter JL (2005) Measurement of the Elastic Modulus of Single Bacterial Cellulose Fibers Using Atomic Force Microscopy. Langmuir 21: 6642-6646.
- Breinl F, Baudisch O (1907) The oxidative breaking up of keratin through treatment with hydrogen peroxide. Z Physiol Chem 52: 158-169.
- Neuberg C (1909) Process of producing digestable subtances from keratin. USA
- Lissizin T (1915) Behavior of keratin sufur and cystin sulfur in the oxidation of these proteins by potassium permaganate I.
- Teslova Zdenko S (1924) Solubility and digestibility of the degradation products of albumoids I. Z Physiol Chem 136: 160-172.
- (1928) The oxidation products of keratin by oxidation withpermagnate II. Z Physiol Chem 173: 309-311.
- Goddard DR, Michaelis L (1935) Derivatives of keratin. J Biol Chem 112: 361-371.
- RochaPlácido Moore G, Maria Martelli S, Gandolfo C, Josédo Amaral Sobral P, Borges Laurindo J (2006) Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocolloids 20: 975-982.
- Goddard DR, Michaelis L (1934) A Study on Keratin. J Biol Chem 106: 605-614.
- Schrooyen PMM, Dijkstra PJ, Oberthür RC, Bantjes A, Feijen J (2001) Stabilization of Solutions of Feather Keratins by Sodium Dodecyl Sulfate. J Colloid Interface Sci 240: 30-39.
- Yamauchi K, Yamauchi A, Kusunoki T, Kohda A, Konishi Y (1996) Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J Biomed Mater Res 31: 439-44.
- Arai KM, Takahashi R, Yokote Y, Akahane K (1983) Amino-Acid Sequence of Feather Keratin from Fowl. Eur J Biochem 132: 501-507.
- Khosa MA, Wu J, Ullah A (2013) Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv.
- Wrześniewska-Tosik KMM, Niekraszewicz A, Potocka DA, Mik T, Pałczyńska M (2011) Fibrous Composites Based on Keratin from Chicken Feathers.Fibres Text East Eur 19: 118-123.
- Ankar CA (1972) Method of preparing keratin-containing films and coatings. US3642498 A.
- Kawano Y, Okamoto S (1975) Film and gels of keratin. Kagaku Seibutsu 13: 291-292.
- Noishiki Y IH, Miyamoto T, Inagaki H (1982) Application of Denatured Wool Keratin Derivatives to an Antithrombogenic Biomaterial-Vascular Graft Coated with a Hyperinized Keratin Derivatives. Kobunshi Ronbunshu 39:249-256.
- Gupta A, Binti KN, Chua YGK, Rosli BMU (2012) Extraction of Keratin Protein from Chicken Feather. J Chem Chem Eng 6: 732-737.
- Carrico IS (2008) Chemoselective modification of proteins: hitting the target. Chem Soc Rev 37:1423-1431.
- Foley TL, Burkart MD (2007) Site-specific protein modification: advances and applications. Curr Opin Chem Biol 11: 12-19.
- Qi D, Tann CM, Haring D, Distefano MD (2001) Generation of new enzymes via covalent modification of existing proteins. Chem Rev 101: 3081-111.
- Davis BG (2004) Mimicking Posttranslational Modifications of Proteins.Science 303: 480-482.
- Crankshaw MW, Grant GA (2001) Modification of Cysteine. Current Protocols in Protein Science, John Wiley & Sons.
- Weerapana E, Simon GM, Cravatt BF (2008) Disparate proteome reactivity profiles of carbon electrophiles. Nat Chem Biol 4: 405-7.
- Chalker JM, Bernardes GJL, Lin YA, Davis BG (2009) Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem Asian J 4: 630-640.
- Clark P, Lowe G (1978) Conversion of the Active-Site Cysteine Residue of Papain into a Dehydro-serine, a Serine and a Glycine Residue. Eur JBiochem 84: 293-299.
- Masri MS, Friedman M (1988) Protein reactions with methyl and ethyl vinyl sulfones. J Protein Chem 7: 49-54.
- Moore JE, Ward WH (1956) Cross-linking of Bovine Plasma Albumin and Wool Keratin. J Am Chem Soc 78: 2414-2418.
- Zhang Y, Gildersleeve J (2012) General Procedure for the Synthesis of Neoglycoproteins and Immobilization on Epoxide-Modified Glass Slides.Methods Mol Biol 808: 155-65.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70-77.
- Anson ML (1940) The Reactions of Iodine and Iodoacetamide with native Egg Albumin. J Gen Physiol 23: 321-31.
- Macindoe WM, Oijen AH, Boons G-J (1998) A unique and highly facile method for synthesising disulfide linked neoglycoconjugates: a new approach for remodelling of peptides and proteins. Chem Commun 7: 847-848.
- Gamblin DP, Garnier P, Kasteren S, Oldham NJ, Fairbanks AJ, et al. (2004) Glyco-SeS: Selenenylsulfide-Mediated Protein Glycoconjugation—A New Strategy in Post-Translational Modification. Angew Chem Int Ed Engl 116: 846-851.
- Dawson P, Muir T, Clark-Lewis I, Kent S (1994) Synthesis of proteins by native chemical ligation. Science 266: 776-779.
- Wan Q, Danishefsky SJ (2007) Free-Radical-Based, Specific Desulfurization of Cysteine: A Powerful Advance in the Synthesis of Polypeptides and Glycopolypeptides. Angew Chem Int Ed 46: 9248-9252.
- Crich D, Brebion F, Krishnamurthy V (2006) Allylic Disulfide Rearrangement and Desulfurization: Mild, Electrophile-Free Thioether Formation from Thiols. Org Lett 8: 3593-3596.
- Crich D, Krishnamurthy V, Hutton TK (2006) Allylic Selenosulfide Rearrangement: A Method for Chemical Ligation to Cysteine and OtherThiols. J Am Chem Soc 128: 2544-2545.
- .Crich D, Krishnamurthy V, Brebion F, Karatholuvhu M, Subramanian V, et al. (2007) Dechalcogenative Allylic Selenosulfide and Disulfide Rearrangements: Complementary Methods for the Formation of Allylic Sulfides in the Absence of Electrophiles. Scope, Limitations, and Application to the Functionalization of Unprotected Peptides in Aqueous Media. J Am Chem Soc 129: 10282-10294.
- Crich D, Yang F (2008) Synthesis of Neoglycoconjugates by the Desulfurative Rearrangement of Allylic Disulfides. J Org Chem 73: 7017-7027.
- Holmes TJ, Lawton RG (1977) Cysteine modification and cleavage of proteins with 2-methyl-N1-benzenesulfonyl-N4-bromoacetylquinonediimide.J Am Chem Soc 99: 1984-1986.
- Jacob C, Giles GI, Giles NM, Sies H (2003) Schwefel und Selen: Bedeutung der Oxidationsstufe für Struktur und Funktion von Proteinen. Angew Chem Int Ed Engl 115:4890-4907.
- Yan LZ, Dawson PE (2001) Synthesis of Peptides and Proteins without Cysteine Residues by Native Chemical Ligation Combined withDesulfurization. J Am Chem Soc 123: 526-533.
- Smith JJ, Conrad DW, Cuneo MJ, Hellinga HW (2005) Orthogonal sitespecific protein modification by engineering reversible thiol protection mechanisms. Protein Sci 14: 64-73.
- Cameron GJ, Wess TJ, Bonser RH (2003) Young’s modulus varies with differential orientation of keratin in feathers. J Struct Biol 143: 118-23.
- Tachibana A, Furuta Y, Takeshima H, Tanabe T, Yamauchi K (2002) Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J Biotechnol 93: 165-70.
- Conte A, Buonocore GG, Bevilacqua A, Sinigaglia M, Del Nobile MA (2006) Immobilization of lysozyme on polyvinylalcohol films for active packaging applications. J Food Prot 69: 866-70.
- Tachibana A, Kaneko S, Tanabe T, Yamauchi K (2005) Rapid fabrication of keratin-hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 26: 297-302.
- Tachibana A, Nishikawa Y, Nishino M, Kaneko S, Tanabe T, et al. (2006) Modified keratin sponge: binding of bone morphogenetic protein-2 andosteoblast differentiation. J Biosci Bioeng 102: 425-9.
- Katoh K, Tanabe T, Yamauchi K (2004) Novel approach to fabricate keratin sponge scaffolds with controlled pore size and porosity. Biomaterials 25: 4255-62.
- Peplow PV, Dias GJ (2004) A study of the relationship between mass andphysical strength of keratin bars in vivo. J Mater Sci Mater Med 15:1217-20.
- Verma V, Verma P, Ray P, Ray AR (2008) Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed Mater 3: 025007.
- Barone JR, Schmidt WF, Liebner CFE (2005) Compounding and molding of polyethylene composites reinforced with keratin feather fiber. Compos Sci Technol 65: 683-692.
- Aluigi A, Varesano A, Montarsolo A, Vineis C, Ferrero F, et al. (2007) Electrospinning of keratin/poly (ethylene oxide) blend nanofibers. J Appl Polym Sci 104: 863-870.
- Aluigi A, Vineis C, Varesano A, Mazzuchetti G, Ferrero F, et al. (2008) Structure and properties of keratin/PEO blend nanofibres. Eur Polym J 44: 2465-2475.
- Varesano A, Aluigi A, Vineis C, Tonin C (2008) Study on the shear viscosity behavior of keratin/PEO blends for nanofibre electrospinning. J Polym Sci Part B: Polym Phys 46:1193-1201.
- Yamauchi K, Yamauchi A, Kusunoki T, Kohda A, Konishi Y (1996) Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J Biomed Mater Res 31:439-444.
- Fujii T, Ogiwara D, Arimoto M (2004) Convenient Procedures for Human Hair Protein Films and Properties of Alkaline Phosphatase Incorporated in the Film. Biol Pharm Bull 27: 89-93.
- Fujii T, Ide Y (2004) Preparation of Translucent and Flexible Human Hair Protein Films and Their Properties. Biol Pharm Bull 27: 1433-1436.
- Katoh K, Shibayama M, Tanabe T, Yamauchi K (2004) Preparation and physicochemical properties of compression-molded keratin films. Biomaterials 25: 2265-2272
- Tanabe T, Okitsu N, Tachibana A, Yamauchi K (2002) Preparation andcharacterization of keratin-chitosan composite film. Biomaterials 23: 817-25.
- Yamauchi K, Hojo H, Yamamoto Y, Tanabe T (2003) Enhanced cell adhesionon RGDS-carrying keratin film. Mater Sci Eng: C 23: 467-472.
- Lee KY, Ha WS (1999) DSC studies on bound water in silk fibroin/Scarboxymethyl kerateine blend films. Polymer 40: 4131-4134.
- Lee KY, Kong SJ, Park WH, Ha WS, Kwon IC (1998) Effect of surface properties on the antithrombogenicity of silk fibroin/S-carboxymethylkerateine blend films. J Biomater Sci Polym Ed 9: 905-14.
- Lee K (2001) Characterization of silk fibroin/S-carboxymethyl kerateine surfaces: Evaluation of biocompatibility by contact angle measurements. Fiber Polym 2: 71-74.
- Vasconcelos A, Freddi G, Cavaco-Paulo A (2008) Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 9: 1299-1305.
- Tonin C, Aluigi A, Vineis C, Varesano A, Montarsolo A, et al. (2007) Thermaland structural characterization of poly (ethylene-oxide)/keratin blend films. J Therm Anal Calorim 89: 601-608.
- Zoccola M, Aluigi A, Vineis C, Tonin C, Ferrero F, et al. (2008) Study on Cast Membranes and Electrospun Nanofibers Made from Keratin/Fibroin Blends. Biomacromolecules 9: 2819-2825.
- Selmin F, Cilurzo F, Aluigi A, Franzè S, Minghetti P (2012) Regenerated keratin membrane to match the in vitro drug diffusion through human epidermis. Results Pharma Sci 2: 72-78.
- Reichl S (2009) Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 30: 6854-66.
- Barone JR, Schmidt WF, Liebner CFE (2005) Thermally processed keratin films. J Appl Polym Sci 97: 1644-1651.
- Ullah A, Wu J (2013) Feather Fiber-Based Thermoplastics: Effects of Different Plasticizers on Material Properties. Macromol Mater Eng 298: 153-162.
- Kar P, Misra M (2004) Use of keratin fiber for separation of heavy metals from water. J Chem Technol Biotechnol 79: 1313-1319.
- Malana MA, Khosa MA (2011) Groundwater pollution with special focus on arsenic, Dera Ghazi Khan-Pakistan. J Saudi Chem Soc 15: 39-47.
- Benhima H, Chiban M, Sinan F, Seta P, Persin M (2008) Removal of lead and cadmium ions from aqueous solution by adsorption onto micro-particles of dry plants. Colloid Surf B Biointerfaces 61: 10-16.
- Khosa MA, Shah SS, Feng X (2013) Micellar Enhanced Ultrafiltration of Organic Dyes. Separ Sci Technol 48: 1315-1323.
- Khosa MA, Shah SS, Nazar MF (2011) Application of Micellar Enhanced Ultrafiltration for the Removal of Methylene Blue from Aqueous Solution. J Disper Sci Technol 32: 260-264.
- Khosa MA, Shah SS, Nazar MF (2011) UV-Visible Spectrometric Study and Micellar Enhanced Ultrafiltration of Alizarin Red S Dye. J Disper Sci Technol 32: 1634-1640.
- Sekhar KC, Subramanian S, Modak JM, Natarajan KA (1998) Removal of metal ions using an industrial biomass with reference to environmentalcontrol. Inter J Miner Process 53: 107-120.
- Hojo N (1958) The change of adsorbability of Hg, Cu, and Ag of wool through alkali treatment. Sen-I Gakkaishi 14: 953-955.
- FR H (1968) Studies in Chrome Mordanting II. The binding of chromium (III) cations to wool. Aust J Chem 21: 2723-2735.
- FR H (1968) The uptake of aluminium by wool. Aust J Chem 21:1013-1022.
- Guthrie RE, Laurie SH (1968) The binding of copper (II) to mohair keratin. Aust J Chem 21: 2437-2443.
- Suyama K, Fukazawa Y, Suzumura H (1996) Biosorption of precious metal ions by chicken feather. Appl Biochem Biotechnol 57-58: 67-74.
- Ishikawa S, Suyama K (1998) Recovery and refining of Au by gold-cyanide ion biosorption using animal fibrous proteins. Appl Biochem Biotechnol 70-72: 719-28.
- Ishikawa SI, Sekine S, Miura N, Suyama K, Arihara K, et al. (2004) Removal of selenium and arsenic by animal biopolymers. Biol Trace Elem Res 102:113-127.
- Stephenson AH, McCaskey TA, Ruffin BG (1990) A survey of broiler litter composition and potential value as a nutrient resource. Biol Wastes 34: 1-9.
- Gupta G, Borowiec J, Okoh J (1997) Toxicity identification of poultry litter aqueous leachate. Poult Sci 76: 1364-7.
- Turan NG (2011) Metal uptake from aqueous leachate of poultry litter by natural zeolite. Environ Prog Sustain Energy 30: 152-159.
- Sun P, Liu ZT, Liu ZW (2009) Chemically Modified Chicken Feather as Sorbent for Removing Toxic Chromium(VI) Ions. Ind Eng Chem Res 48:6882-6889./a>
- Teixeira MC, Ciminelli VS (2005) Development of a biosorbent for arsenite: structural modeling based on X-ray spectroscopy. Environ Sci Technol 39: 895-900.
- Banat F, Al-Asheh S, Al-Rousan D (2002) Comparison between Different Keratin-composed Biosorbents for the Removal of Heavy Metal Ions fromAqueous Solutions. Adsorpt Sci Technol 20: 393-416.
- Al-Asheh S, Banat F, Al-Rousan D (2003) Beneficial reuse of chicken feathers in removal of heavy metals from wastewater. J Clean Prod 11: 321-326.
- Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, et al. (2006) New consensus nomenclature for mammalian keratins. J Cell Biol 174:169-74.
- Zhen LS (2005) Ben Cao Gang Mu:The Time Literature & Art Press:Changchun, Jilin, China.
- Hofmeier J (1905) Horn-lime plastic masses from keratin substances. Patent N DE184915, Germany./a>
- Van den Bergh JM, van Dijk GJ, HEP (1941) Keratin-resin threads, films, etc. Patent N 51000577, Netherland.
- Magin TM, Vijayaraj P, Leube RE (2007) Structural and regulatory functions of keratins. Exp Cell Res 313: 2021-32.
- Izawa I, Inagaki M (2006) Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci 97: 167-74.
- Yamauchi K, Maniwa M, Mori T (1998) Cultivation of fibroblast cells on keratin-coated substrata. J Biomater Sci Polym Ed 9: 259-70.
- Apel PJ, Garrett JP, Sierpinski P, Ma J, Atala A, et al. (2008) Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J Hand Surg Am 33: 1541-7.
- Sierpinski P, Garrett J, Ma J, Apel P, Klorig D, et al. (2008) The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29:118-28.
- Aguayo-Villarreal IA, Bonilla-Petriciolet A, Hernández-Montoya V, Montes-Morán MA, Reynel-Avila HE (2011) Batch and column studies of Zn2+ removal from aqueous solution using chicken feathers as sorbents. Chem Eng J 167: 67-76.
- Ishikawa Si, Suyama K (1998) Recovery and Refining of Au by Gold-Cyanide Ion Biosorption Using Animal Fibrous Proteins. Finkelstein M and Davison B (eds) Biotechnology for Fuels and Chemicals, Humana Press 719-728.
- Senoz E, Stanzione JF, Reno KH, Wool RP, Miller MEN (2013) Pyrolyzed chicken feather fibers for biobased composite reinforcement. J Appl Polym Sci 128: 983-989.
- Naik PSKGR (2010) Production and characterization of feather degrading keratinase from Bacillus sp. JB 99. IJBT 9: 384-390.
- Zhan M, Wool RP (2011) Mechanical properties of chicken feather fibers. Polym Compos 32: 937-944..