Journal of Forensic Investigation
Download PDF
Research Article
Morphometric Analysis of Tongue in Individuals of European and African Ancestry
Marcela Beghini1, Thiago Lima Pereira1, Jean Mateus Cezarine Montes1,Douglas Vieira De Moura 1, Thai Uenoyama Dezem2, Ricardo Henrique Alves Da Silva2, Denise Bertulucci Rocha Rodrigues 1,3 and Sanivia Aparecida De Lima Pereira1,3*
- 1Laboratório de Biologia Celular e Molecular, Universidade de Uberaba (UNIUBE), Brazil
- 2Departamento de Odontologia Forense, Faculdade de Odontologia de Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto, Brazil
- 3Cefores, Universidade Federal do Triangulo Mineiro (UFTM), Uberaba, Brazil
*Address for Correspondence: Sanivia Aparecida De Lima Pereira, Laboratório de Biologia Celular e Molecular, Universidade de Uberaba (UNIUBE), Av. Nenê Sabino, 1801, Bairro Universitário, CEP 38.055-500, Uberaba MG, Brazil, Tel: +55 34991535353; Fax: +55 34 3314 910; E-mail: sanivia.pereira@uniube.br
Citation: Beghini M, Pereira TL, Montes JMC, De Moura DV, Dezem TU, et al. Morphometric Analysis of Tongue in Individuals of European and African Ancestry. J Forensic Investigation. 2017; 5(1): 5.
Copyright: © 2017 De Lima Pereira SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Forensic Investigation | ISSN: 2330-0396 | Volume: 5, Issue: 1
Submission: 02 June, 2017 | Accepted: 05 July, 2017 | Published: 11 July, 2017
Keywords
Autopsy; Forensic dentistry; Tongue; Forensic anthropology; Physical anthropology
Abstract
The maxillary dental arch is generally preserved due to its relatively protected location, as well as due to the strength of the teeth because of cadaveric spasm. However, when the victim is edentulous, or when there are not enough comparative data to effectively analyze the dental arches, human identification information based on forensic dentistry methods is very limited. Therefore, since the tongue is an organ of easy access and presents resistance to decomposition and carbonization because it is located in a humid closed cavity, it has become an important organ in post-mortem evaluation. The objective of study was to compare the human tongue dimensions and collagen percentage according to ancestry, gender and age groups. Forty-five tongues were removed during autopsy performed between 1 and 8 hours after death and immediately fixed in formaldehyde in order to avoid decomposition. The tongue length, width and thickness of autopsied individuals were analyzed. Collagen percentage was determined using AxioVision software on slides stained with Picrosirius. The subjects were classified according to gender, age, and ancestry.The tongues length of African ancestry individuals were significantly longer than European ancestry tongues (p = 0.02). The European descendants showed significantly higher collagen percentage than the African descendants (p = 0.01). There was no significant difference regarding collagen percentage between gender groups or age groups. There were also no significant differences in tongue thickness and width according to the ancestry, gender or age groups. This study is the first to compare the size and collagen percentage of the human tongue between different gender, ethnic and age groups.
In conclusion the African ancestry individuals were significantly longer and the European ancestry tongues had a higher collagen percentage. Therefore we suggested that tongue length and collagen percentage analysis could be used as additional parameter for human identification, especially in cases of quartering or even in high-impact accidents where isolated parts of the human body can be found far from the site of the murder or accident. However, further research are needed to confirm these findings.
Introduction
Human identification is the process of determining the identity of a person, or a set of measures with the purpose of disclosing an identity [1].
The evaluation of bones and teeth is extremely common for the identification of bodies that are burned, decomposed, skeletonized, mutilated or fragmented for any reason. Human identification using bone samples can raise evidence regarding gender, ancestry, and estimated age [2]. There are several gender determination procedures in forensic anthropology that analyze morphological characteristics of the skull, jaw and pelvis [3]. For example, closure of the cranial sutures and dental-alveolar wear provide valuable information about the estimated age of adults [4]. Although these evaluations help determine the gender and age of the individuals, the validity of this approach depends on the condition of the bones and bone fragments [5].
Identification through examination of the dental arches requires analysis of previous dental records of the person suspected, so that the ante-mortem and post-mortem examinations may be contrasted, resulting in a positive or negative identification [2]. The maxillary dental arch is generally preserved due to its relatively protected location, as well as due to the strength of the teeth because of cadaveric spasm [5]. However, when the victim is edentulous, or when there are not enough comparative data to effectively analyze the dental arches, human identification information based on forensic dentistry methods is very limited [6].
Therefore, since the tongue is an organ of easy access and presents resistance to decomposition and carbonization because it is located in a humid closed cavity, it has become an important organ in postmortem evaluation [7,8].
Studies have shown that the organs of the human body are generally larger in male individuals and in African descendants and also that they decrease in size with aging [9-21]. It is also known that individuals of African ancestry generally have higher collagen percentage in skin when compared to individuals of European ancestry [22]. Furthermore, several studies have shown that collagen decreases in several organs with aging [23]. However, we did not find any studies assessing collagen percentage and sizes of the human tongue, or associating it with different age, gender or ethnic groups in the literature to date.
Therefore, the tongue evaluation for the recognition of corpses is important especially in cases of quartering or even in high-impact accidents where isolated parts of the human body can be found far from the site of the murder or accident.
Thus, the aim of this study was to compare the human tongue dimensions and collagen percentage according to ancestry, gender and age groups.
Materials and Methods
Selection of research subjects
After approval by the Ethics Committee on Human Research of the Federal University of Triangulo Mineiro (UFTM), Uberaba, MG, Brazil (protocol number 2635), 44 autopsy protocols were selected at the Clinical Hospital of UFTM. The tongues were removed during an autopsy performed between 1 and 8 hours after death and immediately fixed in formaldehyde in order to avoid decomposition. The individuals were classified according to the following criteria:
a) Gender: Male (n = 29), Female (n = 15);
b) Ethnicity: European ancestry (n = 33), African ancestry (n = 11); and
c) Age: First the subjects were divided into five groups: 20 - 29 years, 30 - 39 years, 40 - 49 years, 50 - 59 years, and 60 - 79 years.
Posteriorly the individuals were divided into three groups: 20 - 39 years, 40 - 59 years, and 60 - 79 years. This grouping in only three groups allowed an increase in the number of cases per group. All individuals of African ancestry were pure.
Morphometric evaluation of the tongues
Morphometric evaluations of the tongues were evaluated length, width and thickness of 44 tongues of necropsied individuals adults previously stored in a 3.7% formaldehyde solution.
In order to evaluate the tongue length, a flexible millimeter ruler was placed along the median lingual sulcus on the tongues lingual dorsum, from the epiglottis to the apex. A digital caliper was used to analyze the width and thickness of the tongues. Ten consecutive measurements of width and thickness were performed from the base to apex of each tongue, and the higher values of width and thickness were used for the statistical analyses. The measurements were expressed in millimeters. During the tongue handling all personal protective equipment like masks, aprons, protective goggles and gloves were used. A single trained, calibrated examiner blindly analyzed the tongues.
Morphometric analysis of collagen
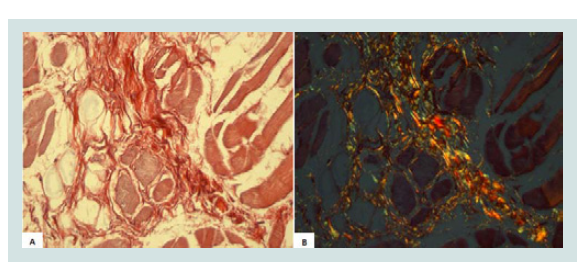
For collagen analysis, a section from the middle region of each tongue was collected, measuring about 1.0 x 1.0 cm wide and 0.5 cm thick. This section was histologically processed and the slides were stained by Picrosirius. For collagen analysis, was used a light biological microscope, Axio 4.1 (ZEISS, Berlin, Germany) and AxioCam camera (ZEISS, Berlin, Germany) for capturing images, a computer and AxioVision 4.8 software (ZEISS, Berlin, Germany) with 40X objective and a polarizing filter. The images shown in the microscope were transmitted to the computer monitor. In the polarized images, collagen birefringence was shown in reddish yellow, and it was automatically quantified (Figures 1A and 1B). All the fields of each fragment, approximately 20 fields per fragment, were analyzed.
Figure 1: A) Collagen in the tongue muscle (Picrosirius - image not polarized, 800X); B) Collagen in the tongue muscle (Picrosirius - polarized image, 800X).
Statistical analyses
GraphPad Prism 4.0 statistical software (GRAPHPAD SOFTWARE, San Diego, USA) was used for the statistical analyses. The Shapiro-Wilk test was used to evaluate the normality. In order to compare the length, width, thickness and collagen percentage of the tongues between European and African ancestry groups and between male and female groups, Student’s t-test was used when quantitative variables showed normal distribution, and the Mann-Whitney test was used when quantitative variables showed nonnormal distribution. The Kruskal-Wallis test was used to compare the age groups with non-normal distribution. Spearman correlation test was used to evaluate the correlation between the ages and collagen percentage. The significance level was 5% (p < 0.05).
Results
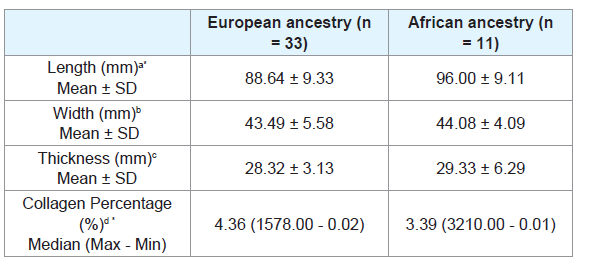
The African ancestry individuals showed significantly longer tongues than the European ancestry individuals (p = 0.02) (Table 1).
Table 1: Length, width, thickness and collagen percentage of tongue of European ancestry and African ancestry subjects.
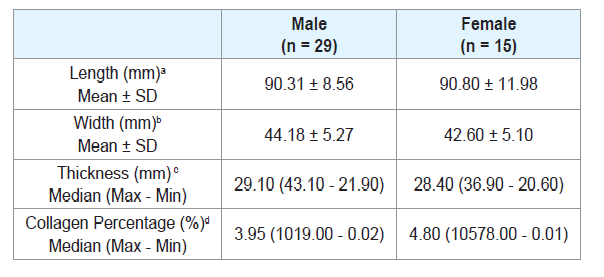
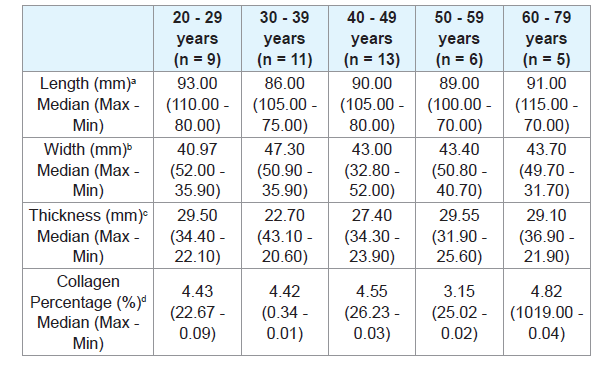
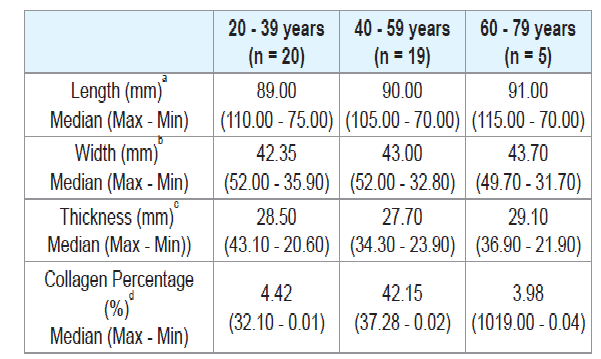
There was no significant difference when comparing the tongue length between males and females (Table 2), or between age groups (Tables 3 and 4). There were also no significant differences in thickness or width of tongues according to the ancestry (Table 1), between males and females (Table 2), and between age groups (Tables 3 and 4).
The European ancestry showed significantly higher collagen percentage than the African ancestry (p = 0.30) (Table 1). There was no significant difference regarding collagen percentage between gender groups (Table 2) or age groups (Tables 3 and 4). There was no significant correlation between collagen percentage and age, and between collagen percentage and tongue width, length and thickness (data not showed).
Discussion
Anthropological patterns have been investigated in order to contribute to anatomical data collection from corpses and skeletons indifferent parts of the world, hence facilitating human identification. These studies are aimed at establishing protocols for determining the gender, age, height, ancestry and individualization factors [24,25]. A preliminary assessment during post-mortem examination allows forensic anthropologists to gather the necessary information for a rough estimate of the actual anatomical parameters of an individual [26,27]. Some studies have demonstrated that African ancestry individuals have a larger bone density [20], larger palatal area [28], increased hip flexion strength [29], greater muscle mass [30,31], higher total-body calcium, phosphorus, sodium, chlorine, and potassium [32], higher lean body mass [33], higher density of dermal cells [22], and numerous superficial vessels on the skin compared to European descendants [34]. Although morphometric studies have not been conducted comparing the dimensions of the human tongue among ethnic groups, we believe that in the present study, the tongues of African ancestry individuals were longer than those of the European ancestry, perhaps because the African ancestry individuals had greater tongue muscle mass as demonstrated in the appendicular muscle in previous studies [30,31]. The greater lingual length in individuals of African ancestry would be justified by the higher levels of testosterone in these individuals, which is a hormone directly associated with muscle hypertrophy.
There was no significant difference in tongue length, width or thickness between men and women. Although studies comparing tongue length, width or thickness between the sexes have not been found, studies have been carried out comparing muscle strength and tongue mobility between female and male individuals [13,31]. However, the difference in lingual muscle strength between genders is still controversial as it has already been reported greater strength in the male tongue, although no differences were found in another study [35,36]. It is also known that women have a higher percentage of muscle fibers in the middle region of the tongue, which gives greater muscle mobility [37]. Since the previous studies evaluated only the muscular strength and mobility of the tongue, the present study was the first to compare tongue length, width and thickness between male and female individuals.
It is known that the axial force of the tongue increases during childhood and adolescence, and stabilizes in adulthood [38]. However, some studies in living individuals showed that the muscle strength of the tongue reduces after 60 years due to decreased muscle mass and motor units and due to decreased in the density of the muscle fibers [36,38-42]. Only one study that evaluated the thickness of the human tongue according to age groups was found in the literature. In this study, the authors observed in vivo that elderly individuals had lesser tongue thickness compared to young individuals [38]. However in the present study there was no significant difference in the length, width and thickness of the tongues when comparing different age.
Although it has been described in the literature that individuals of African ancestry have a greater amount of collagen in several tissues, in the present study, the tongues of African ancestry individuals had a lower percentage of collagen than the tongues of European ancestry individuals [22]. It is known that in the tissues of African ancestry individuals the bundles of collagen are smaller, there is a greater amount of glycoproteins in the dermal interstitium, and the macrophages are larger and more numerous than in European ancestry individuals [22,43]. Therefore, in African ancestry individuals the collagen bundles would be more spaced, being intercalated by the other components of the extracellular matrix, which could justify the fact that we found a lower percentage of collagen in this group, since this percentage of collagen, was evaluated by area. The present study is the first study to compare the size and collagen percentage of the human tongue between different gender, ethnic and age groups.
In conclusion the African ancestry individuals were significantly longer and the European ancestry tongues had a higher collagen percentage. Therefore we suggested that tongue length and collagen percentage analysis could be used as additional parameter for human identification, especially in cases of quartering or even in high-impact accidents where isolated parts of the human body can be found far from the site of the murder or accident. However, furthers research are needed to confirm these findings.
Acknowledgement
We thank the Disciplina de Patologia Geral/UFTM, Programa de Pós Graduação em Odontologia da Universidade de Uberaba/UNIUBE, Departamento de Odontologia Forense da Faculdade de Odontologia de Ribeirão Preto (USP), Cefores/Universidade Federal do Triângulo Mineiro (UFTM), Programa de Pós Graduação em Ciências da Saúde/ UFTM; Fundação de Apoio à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), andtheFundação de Educação e Pesquisa de Uberaba (FUNEPU).
References
- França G (2008) Legal Medicine. Guaranabara Koogan, Rio de Janeiro, Brasil, Portuguese.
- Tornavoi DC, da Silva RH (2010) Palatal rugae and applicability in human identification in forensic dentistry: literature review. Saúde Ética Justiça 15: 28-34.
- Suazo Galdames IC, Zavando Matamala DA, Smith RL (2009) Performance evaluation as a diagnostic test for traditional methods for forensic identification of sex. Int J Morphol 27: 381-386.
- Galdames IS, Flores A, Roa I, Cantín M, Zavando D (2011) Sex determination by observation of barr body in teeth subjected to high temperatures. Int J Morphol 29: 199-203.
- Gajardo P, Gajardo M, Torres S, Zavando D, Galdames IS (2011) Determination of stature from the bow and maxillo-rope: stature determination from maxillary arch and radio-cord. Int J Odontostomat 5: 267-269.
- Dawasaz AA, Dinkar AD (2013) Rugoscopy: predominant pattern, uniqueness, and stability assessment in the Indian Goan population. J Forensic Sci 58: 1621-1627.
- de Lima Pereira SA, Severino VO, Kohl NL, Rodrigues DB, Alves PM, et al. (2009) Expression of cytokines and chemokines and microvasculature alterations of the tongue from patients with chronic Chagas’ disease. Parasitol Res 105: 1031-1039.
- Gould GA (2004) Forensic odontology: a global activity. J Calif Dent Assoc 32: 410-415.
- Teixeira VP, Rodrigues DB, Reis MA, Castro EC, Piccioni DE, et al. (2013) Comparison of the total length and areas of upper central incisors between males and females using computer-assisted morphometry. Anat Sci Int 88: 130-133.
- Manoel C, Prado FB, Caria PH, Groppo FC (2009) Morphometric analysis of the foramen magnum in human skulls of brazilian individuals: its relation to gender. J Morphol Sci 26: 104-108.
- Sato H, Sando I, Takahashi H (1991) Sexual dimorphism and development of the human cochlea. Computer 3-D measurement. Acta Otolaryngol 111: 1037-1040.
- Ruff C (1987) Sexual dimorphism in human lower limb bone structure: relationship to subsistence strategy and sexual division of labor. J Hum Evol 16: 391-416.
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL (1997) Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry 21: 1185-1201.
- Samal A, Subramani V, Marx D (2007) Analysis of sexual dimorphism in human face. J Vis Commun Image Represent 18: 453-463.
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, et al. (2001) Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11: 490-497.
- Wells JC (2007) Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 21: 415-430.
- Brace CL, Ryan AS (1980) Sexual dimorphism and human tooth size differences. J Hum Evol 9: 417-435.
- Ortiz O, Russell M, Daley TL, Baumgartner RN, Waki M, et al. (1992) Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 55: 8-13.
- Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, et al. (1997) Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 83: 229-239.
- Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, et al. (2007) Race/ethnic differences in bone mineral density in men. Osteoporos Int 18: 943-953.
- Kumar V, Abbas AK, Aster JC (2015) Robbins & Cotran pathologic basis of disease, (9thedn). Elsevier, Brazil, pp. 1408.
- Montagna W, Carlisle K (1991) The architecture of black and white facial skin. J Am Acad Dermatol 24 (6 Pt 1): 929-937.
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, et al. (2006) Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol 168: 1861-1868.
- Cattaneo C (2007) Forensic anthropology: developments of a classical discipline in the new millennium. Forensic Sci Int 165: 185-193.
- Işcan MY (2001) Global forensic anthropology in the 21st century. Forensic Sci Int 117: 1-6.
- Rothwell BR (2001) Principles of dental identification. Dent Clin North Am 45: 253-270.
- Jurado J, Martínez JM, Quenguán R, Martinez C, Moreno F (2009) Analysis of palatal rugae in young persons to two Colombian ethnic groups. Rev Estomat 17: 17-22.
- Olze A, Reisinger W, Geserick G, Schmeling A (2006) Age estimation of unaccompanied minors. Part II. Dental aspects. Forensic Sci Int 159 Suppl 1: S65-S67.
- Rantanen T, Guralnik JM, Leveille S, Izmirlian G, Hirsch R, et al. (1998) Racial differences in muscle strength in disabled older women. J Gerontol A Biol Sci Med Sci 53: B355-B361.
- Aloia JF, Vaswani A, Ma R, Flaster E (1997) Comparison of body composition in black and white premenopausal women. J Lab Clin Med 129: 294-299.
- Gasperino JA, Wang J, Pierson RN Jr, Heymsfield SB (1995) Age-related changes in musculoskeletal mass between black and white women. Metabolism 44: 30-34.
- Cohn SH, Abesamis C, Zanzi I, Aloia JF, Yasumura S, et al. (1977) Body elemental composition: comparison between black and white adults. Am J Physiol 232: E419-E422.
- Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, et al. (1984) Density of lean body mass is greater in blacks than in whites. J Appl Physiol Respir Environ Exerc Physiol 56: 1647-1649.
- Wesley NO, Maibach HI (2003) Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol 4: 843-860.
- Trawitzki LV, Borges CG, Giglio LD, Silva JB (2011) Tongue strength of healthy young adults. J Oral Rehabil 38: 482-486.
- Youmans SR, Youmans GL, Stierwalt JA (2009) Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia 24: 57-65.
- de Campos D, Jotz GP, Heck L, Xavier LL (2014) Sexual dimorphism in the histologic organization of the muscle fibers in human tongue. J Voice 28: 424-429.
- Potter NL, Short R (2009) Maximal tongue strength in typically developing children and adolescents. Dysphagia 24: 391-397.
- McAuliffe MJ, Ward EC, Murdoch BE, Farrell AM (2005) A nonspeech investigation of tongue function in Parkinson's disease. J Gerontol A Biol Sci Med Sci 60: 667-674.
- Mortimore IL, Bennett SP, Douglas NJ (2000) Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res 9: 389-393.
- Robbins JA, Levine R, Wood J, Roecker EB, Luschei E (1995) Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci 50: M257-M262.
- Crow HC, Ship JA (1996) Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci 51: M247-M250.
- Alchorne MM, De Abreu MA (2008) Dermatology in black skin. An Bras Dermatol 83.