Journal of Cytology & Molecular Biology
Download PDF
Research Article
*Address for Correspondence: Connie Darmanin, ARC Centre of Advanced Molecular Imaging, Department of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia, Tel: 61-3-94679-1329; Fax: 61-3-9479-1552; E-mail: c.darmanin@latrobe.edu.au
Citation: Darmanin C, Liang YL. Reconstitution of GPCRs in Lipidic Cubic Phase (LCP): Comparison between Human Histamine 1 and Dopamine 2 Long Receptor Reconstitution into Five Different Self-Assembly Lipids. J Cytol Molecul Biol. 2015; S(1): 6.
Copyright © 2015 Darmanin C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cytology & Molecular Biology | ISSN: 2325-4653 | Special Issue: 1
Submission: 21 October 2015 | Accepted: 10 December 2015 | Published: 15 December 2015
The success of in meso crystallization is the result of the lipidic cubic phase (LCP) introduction to the crystallization process. LCP lipids are self-assembly lipids that have the unique properties and are closely related to the native lipid environment of a cell. They contain two domains which consist of the hydrophobic bilayer that accommodates the hydrophobic portion of the receptor and interconnecting water channels, which satisfy the hydrophilic portions of the receptor. The three-dimensional complexity of this lipid system allows the GPCR to reconstitute itself into the selfassembly lipid relatively easily. However, there are two restrictions of the LCP environment that affects the uptake of the receptor so that it remains in a functional state; bilayer thickness and water channel diameter. The size of these domains determine the lipids compatibility with the GPCR of interest and hence determines the phase of the lipid upon reconstitution in the initial phase of crystallization.
In 2008, Caffrey proposed a mechanism for crystallizing proteins using LCP [3]. It was suggested that the addition of precipitants such as polymers, polyols, and salts cause the cubic phase to become less stable thereby resulting in a phase shift and subsequent phase separation [3]. In order for crystallization to be successful, it is predicted the membrane proteins reconstitute into the LCP where crystal nucleation occurs followed by a lipid transition to a lamellar phase where crystal growth proceeds [3]. Provided the composition of the solution and structure of the bilayer are appropriate, a high concentration of protein should result in nucleation followed by crystal growth [3]. Therefore the initial lipid phase is critical for successful LCP crystallization. Currently, the most commonly used LCP lipid for in meso crystallization is monoolein (MO) and its derivatives. It has been successful in crystallizing a number of GPCRs [4-6]. However, we have shown in a previous study, using small angle x-ray scattering measurements, that MO is unable to uptake more than ~2 mg/ml of Dopamine 2 long receptor (D2L) into the lipid before a phase change occurs in the system [7]. At this concentration this reduces the likelihood of D2L crystallization and therefore the need to look for alternative LCP systems is prevalent. In addition incorporation of additives such as cholesterol has improved LCP crystallization experiments and resulted in a number of solved structures and therefore will also be investigated [8-10].
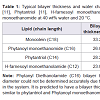
In order to investigate the effect of bilayer thickness and water channel size on the reconstitution of functional GPCRs, we have reconstituted two native GPCRs, Histamine 1 receptor (H1R) and Dopamine 2 long receptor (D2L), into five different selfassembly lipids; Monoolien (MO), Phytantriol (PT), Phytanoyl monoethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE). Table 1 shows the bilayer thickness and water channel sizes of the lipids investigated. GPCR functionality assays were carried out to assess successful reconstitution of the receptor into the lipid systems [11]. We have demonstrated that the bilayer thickness and water channel size of the self-assembly lipid affects the reconstitution of a GPCR and this may affect which lipids to be use for LCP crystallization trials.
Dopamine 2 Long receptor (D2L) and Histamine 1 receptor (H1r) expression and purification
Full length human D2L and H1R receptors were expressed in Sf21 and Sf9 cells, respectively. The receptors were purified using metal affinity columns as described in Darmanin et al. [13]. The purity of the proteins was checked by SDS-PAGE and via Western blot. The concentration of D2L receptor was determined according to Bradford’s method using bovine serum albumin as a standard [14] and using a nanodrop system at wavelength 280 nm on the purified samples.
Radio-ligand binding assay
Functional studies on purified receptor were carried out using either [3H]-Spiperone antagonist (for D2L) or [3H]-Pyrilamine agonist (for H1R) binding assays as outlined in Darmanin et al. [13]. Briefly, Millipore MultiScreen HTS 96-Well filter plates were used for the [3H]-ligand binding assays. The lipids were made up to 200 mg/ml in ethanol and 5 μl of lipid solution (1 mg) was deposited into each well. The plates were allowed to air dry for a further day before aqueous phase (protein solution) was added. 0.69 μl of purified receptor (aqueous) was dispensed on top of the dried lipid film in each well so that the volume of protein added was at a 60:40 ratio for lipid to protein. This was immediately followed by deposition of TMN buffer (50 mM Tris, 10 mM MgCl2, 100 mM NaCl, pH 7.6) and incubation of the samples in a 27 °C water bath, shaking for 1 hour, followed by rapid filtration over 96 well filtration plate system (Millipore) for the lipid assays. The soluble receptor was precipitated with PEG400 on ice for a further 15 minutes before filtration. Nonspecific ligand binding was determined by addition of unlabelled ligand to the reaction mix in alternative wells. The total volume of the assay was 50 μl. Figure 1: Spiperone binding assays showing Dopamine 2L receptor reconstitution into five different lipid systems. Two different D2L concentrations were tested (A) 5 mg/ml and (B) 3 mg/ml in five different self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide(FE) and Phytanyl Diethanolamide (PDiE). Ultima Gold™ (Perkin Elmer) liquid scintillant (50 μl) was added to each filter plate well and beta radiation was detected in a Wallac MicroBeta TriLux 1450 LSC and Luminescence Counter (Perkin Elmer). Binding data was evaluated by a nonlinear, least squares curve-fitting procedure using GRAPHPAD PRISM4 (GRAPHPAD Software, Inc, San Diego, CA, USA). The ligand affinities were calculated according to Swillens by using a global fitting procedure to determine total and nonspecific binding at the same time [15]. Nonspecific binding was determined by saturation binding experiments, which estimated nonspecific binding under total binding conditions.
Figure 1: Spiperone binding assays showing Dopamine 2L receptor reconstitution into five different lipid systems. Two different D2L concentrations were tested (A) 5 mg/ml and (B) 3 mg/ml in five different self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide(FE) and Phytanyl Diethanolamide (PDiE). Ultima Gold™ (Perkin Elmer) liquid scintillant (50 μl) was added to each filter plate well and beta radiation was detected in a Wallac MicroBeta TriLux 1450 LSC and Luminescence Counter (Perkin Elmer). Binding data was evaluated by a nonlinear, least squares curve-fitting procedure using GRAPHPAD PRISM4 (GRAPHPAD Software, Inc, San Diego, CA, USA). The ligand affinities were calculated according to Swillens by using a global fitting procedure to determine total and nonspecific binding at the same time [15]. Nonspecific binding was determined by saturation binding experiments, which estimated nonspecific binding under total binding conditions.
The purified D2L receptor was reconstituted into five different self-assembly lipids; Monoolien (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) at two different concentration 3 and 5 mg/ ml of receptor. Figure 1 shows at 3 mg/ml, the D2L functionality was at its highest for all of the lipid systems tested when compared to reconstitution experiments at 5 mg/ml for D2L. At 3 mg/ml D2L showed increased functionality by 2.5-, 3.2-, 2-, 2.6-fold for MO, PT, PE and PDiE, respectively, upon reconstitution into the lipid systems, while FE showed no improvement (Figure 1A and 1B ) compared to the 5 mg/ml D2L.
Effect of doping cholesterol into the monoolein with respectto Dopamine 2L receptor
Cholesterol has proven to be a valuable additive for LCP crystallization experiments. Figure 2 shows the addition of cholesterol to MO improves the reconstitution of functional D2L receptor. Our results indicated that the addition of the cholesterol to MO improved the reconstitution of the functional D2L receptor in all three concentrations of cholesterol (5, 7.5 and 10%) tested. However, MO doped with 7.5% cholesterol showed the greatest improvement in functionality which resulted in a 4.5-fold increase in D2L functionality compared to MO (Figure 2). Figure 2: Spiperone binding assays showing reconstitution of the Dopamine 2L receptor Monoolein (MO) doped with cholesterol (Chol). A range of cholesterol percentages were tested, 5%, 7.5% and 10%, in MO to test for improved D2L functionality after reconstitution within the lipid. The D2L concentration used here was 6.8 mg/ml.
Figure 2: Spiperone binding assays showing reconstitution of the Dopamine 2L receptor Monoolein (MO) doped with cholesterol (Chol). A range of cholesterol percentages were tested, 5%, 7.5% and 10%, in MO to test for improved D2L functionality after reconstitution within the lipid. The D2L concentration used here was 6.8 mg/ml.
Comparison of two GPCRs, native Dopamine 2L and Histamine 1 receptor reconstitution into different selfassembly lipids
To compare the effect of reconstituting different GPCRs into self assembly lipids we looked at Histamine 1 receptor (H1R) and compared it to the Dopamine 2L receptor (D2L) results at 5 mg/ ml receptor concentration (Figure 3). The two receptors behaved differently in the five self-assembly lipid systems tested. Generally, the H1R reconstituted slightly more efficiently into all lipid systems tested compared to the D2L receptor (Figure 3A and 3B ). The most efficient self-assembly lipid for both receptors was found to be PDiE. PT and PDiE were shown to be optimal for D2L at 5 mg/ml showing the highest level of functional receptor in the assay after reconstitution into this lipid system, followed by PE, MO and FE (Figure 3A ). The most suitable lipid to increase the functionality of the H1R and hence suitable for reconstitution into a more native state was PDiE, followed by PE, PT and MO/FE (Figure 3B ). Figure 3: Ligand binding assays for two different GPCRs reconstituted into different self-assembly lipids. Five self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) were used for reconstituting (A) D2L and (B) H1R. Both receptor concentrations were at 5 mg/ml.
Figure 3: Ligand binding assays for two different GPCRs reconstituted into different self-assembly lipids. Five self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) were used for reconstituting (A) D2L and (B) H1R. Both receptor concentrations were at 5 mg/ml.
Our results also showed that lipids with a short carbon chain and smaller lipid architecture (bilayer thickness and water channel size), when compared to MO (Table 1), reconstituted both GPCRs more favorably (with the exception of FE). Both PE and PT have a reduced bilayer thickness and water channel size when compared to MO but they produced the best results for reconstituting the native form of the D2L and H1R. Interestingly, PDiE also showed the greatest functionality for both receptors. Phytanyl Diethanolamide (PDiE) is a combination of PE and PT where it contains the chemically robust, isoprenoid-like, branched chain found in PT (C16 and C14, respectively) but contains a diethanolamide head group similar to PE. Therefore it’s not surprising that it improved receptor reconstitution for both H1R and D2L given that its lipid architecture would be a mixture of these lipids.
A number of successful LCP crystallization trials included the addition of cholesterol. The addition of cholesterol into the MO lipid system showed improvement in the reconstitution of D2L. This shows that cholesterol maybe an important additive for crystallizing the native form of the D2L receptor. However, further investigation into the effect of cholesterol in the other self-assembly lipids are required and are currently under investigation in our group.
In summary the self-assembly lipids with the smaller lipid architecture seem to favour the reconstitution of native D2L and H1R. The addition of cholesterol to MO also improved the functional reconstitution of D2L. Therefore PDiE, PE and PT in combination with cholesterol may be the ideal test lipids for crystallizing the native form of D2L and H1R receptors.
Reconstitution of GPCRs in Lipidic Cubic Phase (LCP): Comparison between Human Histamine 1 and Dopamine 2 Long Receptor Reconstitution into Five Different Self-Assembly Lipids
Connie Darmanin1,2* and Yi-Lynn Liang3
- 1ARC Centre of Advanced Molecular Imaging, Department of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria, Australia
- 2CSIRO Materials Science and Engineering (CMSE), Victoria, Australia
- 3Monash University, Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Victoria, Australia
*Address for Correspondence: Connie Darmanin, ARC Centre of Advanced Molecular Imaging, Department of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia, Tel: 61-3-94679-1329; Fax: 61-3-9479-1552; E-mail: c.darmanin@latrobe.edu.au
Citation: Darmanin C, Liang YL. Reconstitution of GPCRs in Lipidic Cubic Phase (LCP): Comparison between Human Histamine 1 and Dopamine 2 Long Receptor Reconstitution into Five Different Self-Assembly Lipids. J Cytol Molecul Biol. 2015; S(1): 6.
Copyright © 2015 Darmanin C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cytology & Molecular Biology | ISSN: 2325-4653 | Special Issue: 1
Submission: 21 October 2015 | Accepted: 10 December 2015 | Published: 15 December 2015
Abstract
G protein-coupled receptors (GPCRs) are key pharmaceutical targets in a number of biological diseases and until the recent introduction of Lipidic Cubic Phase (LCP) crystallization, these protein structure have been extremely difficult to solve. LCP crystallization has been successful in solving the structures of a number of GPCRs. However, understanding the process of LCP crystallization is limited. We have studied reconstitution of two native GPCRs, Dopamine 2 Long receptor and Histamine 1 receptor in five different self-assembly lipids; Monoolien (MO), Phytantriol (PT), Phytanoyl monoethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE). Lipids with the smallest architecture (bilayer thickness and water channel domain) were found to reconstitute these receptors more favourably while MO, the most commonly used lipid in LCP crystallization, was ranked second lowest. We also found incorporation of cholesterol into MO improved GPCR reconstitution.Keywords
GPCRs; Dopamine receptor; Histamine receptor; LCP crystallization; Self-assembly lipids; Cholesterol; MonooleinIntroduction
In meso crystallization has been a successful technique for crystallizing GPCRs, with over 33 unique GPCRs being solved as of October 2015, using this method (http://cherezov.usc.edu/gpcrs.htm). The Stevenson lab led the way in crystallization of GPCRs in 2007, by engineering constructs with a modified intercellular third loop (ICL3) region. The ICL3 is notorious for its high flexibility and movement preventing crystallization. The beta 2-adrenergic receptor was the first to replace the ICL3 loop region, with a modified T4- Lysozyme [1]. This lead the way for an explosive 8 years, where the number of unique structures deposited in the membrane protein data bank soared from 10 to 81 structures between 2007 to 2015 [2]. However, for the Dopamine 2 receptors (long and short) the ICL3 is the only difference between these two isoforms and therefore replacing the ICL3 in this case, is not beneficial for structure-based drug design or understanding the diseases associated with the Dopamine 2 receptors. Therefore understanding the reconstitution step of GPCRs into the LCP lipids will help improve the success rate of in meso crystallization for the native forms of the Dopamine and Histamine receptors.The success of in meso crystallization is the result of the lipidic cubic phase (LCP) introduction to the crystallization process. LCP lipids are self-assembly lipids that have the unique properties and are closely related to the native lipid environment of a cell. They contain two domains which consist of the hydrophobic bilayer that accommodates the hydrophobic portion of the receptor and interconnecting water channels, which satisfy the hydrophilic portions of the receptor. The three-dimensional complexity of this lipid system allows the GPCR to reconstitute itself into the selfassembly lipid relatively easily. However, there are two restrictions of the LCP environment that affects the uptake of the receptor so that it remains in a functional state; bilayer thickness and water channel diameter. The size of these domains determine the lipids compatibility with the GPCR of interest and hence determines the phase of the lipid upon reconstitution in the initial phase of crystallization.
In 2008, Caffrey proposed a mechanism for crystallizing proteins using LCP [3]. It was suggested that the addition of precipitants such as polymers, polyols, and salts cause the cubic phase to become less stable thereby resulting in a phase shift and subsequent phase separation [3]. In order for crystallization to be successful, it is predicted the membrane proteins reconstitute into the LCP where crystal nucleation occurs followed by a lipid transition to a lamellar phase where crystal growth proceeds [3]. Provided the composition of the solution and structure of the bilayer are appropriate, a high concentration of protein should result in nucleation followed by crystal growth [3]. Therefore the initial lipid phase is critical for successful LCP crystallization. Currently, the most commonly used LCP lipid for in meso crystallization is monoolein (MO) and its derivatives. It has been successful in crystallizing a number of GPCRs [4-6]. However, we have shown in a previous study, using small angle x-ray scattering measurements, that MO is unable to uptake more than ~2 mg/ml of Dopamine 2 long receptor (D2L) into the lipid before a phase change occurs in the system [7]. At this concentration this reduces the likelihood of D2L crystallization and therefore the need to look for alternative LCP systems is prevalent. In addition incorporation of additives such as cholesterol has improved LCP crystallization experiments and resulted in a number of solved structures and therefore will also be investigated [8-10].
In order to investigate the effect of bilayer thickness and water channel size on the reconstitution of functional GPCRs, we have reconstituted two native GPCRs, Histamine 1 receptor (H1R) and Dopamine 2 long receptor (D2L), into five different selfassembly lipids; Monoolien (MO), Phytantriol (PT), Phytanoyl monoethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE). Table 1 shows the bilayer thickness and water channel sizes of the lipids investigated. GPCR functionality assays were carried out to assess successful reconstitution of the receptor into the lipid systems [11]. We have demonstrated that the bilayer thickness and water channel size of the self-assembly lipid affects the reconstitution of a GPCR and this may affect which lipids to be use for LCP crystallization trials.
Materials and Methods
Monoolein (M7765) and cholesterol (C8667) were purchased from Sigma-Aldrich. 3,7,11,15-Tetramethyl-1,2,3-hexadecanetriol (phytantriol) was provided by DSM Nutritional Products, Germany. Phytanoyl ethanomide, Phytanyl Diethanolamide and H-farnesoyl monoethanolamide were kindly supplied by Dr. Charlotte Conn (RMIT) [12]. All chemicals unless otherwise state were purchased through Sigma-Aldrich. Tritiated ligands were purchased from Perkin-Elmer.Dopamine 2 Long receptor (D2L) and Histamine 1 receptor (H1r) expression and purification
Full length human D2L and H1R receptors were expressed in Sf21 and Sf9 cells, respectively. The receptors were purified using metal affinity columns as described in Darmanin et al. [13]. The purity of the proteins was checked by SDS-PAGE and via Western blot. The concentration of D2L receptor was determined according to Bradford’s method using bovine serum albumin as a standard [14] and using a nanodrop system at wavelength 280 nm on the purified samples.
Radio-ligand binding assay
Functional studies on purified receptor were carried out using either [3H]-Spiperone antagonist (for D2L) or [3H]-Pyrilamine agonist (for H1R) binding assays as outlined in Darmanin et al. [13]. Briefly, Millipore MultiScreen HTS 96-Well filter plates were used for the [3H]-ligand binding assays. The lipids were made up to 200 mg/ml in ethanol and 5 μl of lipid solution (1 mg) was deposited into each well. The plates were allowed to air dry for a further day before aqueous phase (protein solution) was added. 0.69 μl of purified receptor (aqueous) was dispensed on top of the dried lipid film in each well so that the volume of protein added was at a 60:40 ratio for lipid to protein. This was immediately followed by deposition of TMN buffer (50 mM Tris, 10 mM MgCl2, 100 mM NaCl, pH 7.6) and incubation of the samples in a 27 °C water bath, shaking for 1 hour, followed by rapid filtration over 96 well filtration plate system (Millipore) for the lipid assays. The soluble receptor was precipitated with PEG400 on ice for a further 15 minutes before filtration. Nonspecific ligand binding was determined by addition of unlabelled ligand to the reaction mix in alternative wells. The total volume of the assay was 50 μl.
 Figure 1: Spiperone binding assays showing Dopamine 2L receptor reconstitution into five different lipid systems. Two different D2L concentrations were tested (A) 5 mg/ml and (B) 3 mg/ml in five different self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide(FE) and Phytanyl Diethanolamide (PDiE).
Figure 1: Spiperone binding assays showing Dopamine 2L receptor reconstitution into five different lipid systems. Two different D2L concentrations were tested (A) 5 mg/ml and (B) 3 mg/ml in five different self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide(FE) and Phytanyl Diethanolamide (PDiE). Results
Reconstitution of Dopamine 2L receptor in LCP lipidsThe purified D2L receptor was reconstituted into five different self-assembly lipids; Monoolien (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) at two different concentration 3 and 5 mg/ ml of receptor. Figure 1 shows at 3 mg/ml, the D2L functionality was at its highest for all of the lipid systems tested when compared to reconstitution experiments at 5 mg/ml for D2L. At 3 mg/ml D2L showed increased functionality by 2.5-, 3.2-, 2-, 2.6-fold for MO, PT, PE and PDiE, respectively, upon reconstitution into the lipid systems, while FE showed no improvement (Figure 1A and 1B ) compared to the 5 mg/ml D2L.
Effect of doping cholesterol into the monoolein with respectto Dopamine 2L receptor
Cholesterol has proven to be a valuable additive for LCP crystallization experiments. Figure 2 shows the addition of cholesterol to MO improves the reconstitution of functional D2L receptor. Our results indicated that the addition of the cholesterol to MO improved the reconstitution of the functional D2L receptor in all three concentrations of cholesterol (5, 7.5 and 10%) tested. However, MO doped with 7.5% cholesterol showed the greatest improvement in functionality which resulted in a 4.5-fold increase in D2L functionality compared to MO (Figure 2).
 Figure 2: Spiperone binding assays showing reconstitution of the Dopamine 2L receptor Monoolein (MO) doped with cholesterol (Chol). A range of cholesterol percentages were tested, 5%, 7.5% and 10%, in MO to test for improved D2L functionality after reconstitution within the lipid. The D2L concentration used here was 6.8 mg/ml.
Figure 2: Spiperone binding assays showing reconstitution of the Dopamine 2L receptor Monoolein (MO) doped with cholesterol (Chol). A range of cholesterol percentages were tested, 5%, 7.5% and 10%, in MO to test for improved D2L functionality after reconstitution within the lipid. The D2L concentration used here was 6.8 mg/ml.To compare the effect of reconstituting different GPCRs into self assembly lipids we looked at Histamine 1 receptor (H1R) and compared it to the Dopamine 2L receptor (D2L) results at 5 mg/ ml receptor concentration (Figure 3). The two receptors behaved differently in the five self-assembly lipid systems tested. Generally, the H1R reconstituted slightly more efficiently into all lipid systems tested compared to the D2L receptor (Figure 3A and 3B ). The most efficient self-assembly lipid for both receptors was found to be PDiE. PT and PDiE were shown to be optimal for D2L at 5 mg/ml showing the highest level of functional receptor in the assay after reconstitution into this lipid system, followed by PE, MO and FE (Figure 3A ). The most suitable lipid to increase the functionality of the H1R and hence suitable for reconstitution into a more native state was PDiE, followed by PE, PT and MO/FE (Figure 3B ).
 Figure 3: Ligand binding assays for two different GPCRs reconstituted into different self-assembly lipids. Five self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) were used for reconstituting (A) D2L and (B) H1R. Both receptor concentrations were at 5 mg/ml.
Figure 3: Ligand binding assays for two different GPCRs reconstituted into different self-assembly lipids. Five self-assembly lipids; Monoolein (MO), Phytantriol (PT), Phytanoyl ethanomide (PE), H-farnesoyl monoethanolamide (FE) and Phytanyl Diethanolamide (PDiE) were used for reconstituting (A) D2L and (B) H1R. Both receptor concentrations were at 5 mg/ml. Discussion
The initial concentration of receptor plays a significant role in reconstitution of the GPCR into the lipids. A decrease in receptor concentration from 5 mg/ml to 3 mg/ml for the D2L showed an increase in functional receptor for all lipid systems tested (with the exception of FE) supporting the fact that the GPCR is correctly folding and reconstituting into the lipid system. These two concentrations were initially tested because we previously published results showing a lipid phase change occurred for MO when reconstituted with 1.1 to 4.4 mg/ml of D2L [7]. The phase changed that occurred between these concentrations was cubic diamond to cubic gyroid. The difference between these two phases is the size of the water channels, where the cubic diamond displays a larger water channel size [7]. Ligand binding assays show that around the expected phase change for the D2 receptor in the MO lipid system, functionality is affected. This could mean the receptor is at its optimal reconstitution concentration in the lipid system at 3 mg/ml when MO exists in the cubic diamond phase. Any further addition of receptor may cause a phase change to cubic gyroid in MO resulting in destabilization of the receptor asseen in our previous small angle X-ray studies [7], thereby decreasing the functional receptor reconstitution. The other possibility could be the receptor is continually reconstituted into the lipid system above 3 mg/ml D2L concentration but exists as both as a functional (correctly folded) and non-functional (incorrectly fold) form. This is currently the focus of another publication.Our results also showed that lipids with a short carbon chain and smaller lipid architecture (bilayer thickness and water channel size), when compared to MO (Table 1), reconstituted both GPCRs more favorably (with the exception of FE). Both PE and PT have a reduced bilayer thickness and water channel size when compared to MO but they produced the best results for reconstituting the native form of the D2L and H1R. Interestingly, PDiE also showed the greatest functionality for both receptors. Phytanyl Diethanolamide (PDiE) is a combination of PE and PT where it contains the chemically robust, isoprenoid-like, branched chain found in PT (C16 and C14, respectively) but contains a diethanolamide head group similar to PE. Therefore it’s not surprising that it improved receptor reconstitution for both H1R and D2L given that its lipid architecture would be a mixture of these lipids.
A number of successful LCP crystallization trials included the addition of cholesterol. The addition of cholesterol into the MO lipid system showed improvement in the reconstitution of D2L. This shows that cholesterol maybe an important additive for crystallizing the native form of the D2L receptor. However, further investigation into the effect of cholesterol in the other self-assembly lipids are required and are currently under investigation in our group.
In summary the self-assembly lipids with the smaller lipid architecture seem to favour the reconstitution of native D2L and H1R. The addition of cholesterol to MO also improved the functional reconstitution of D2L. Therefore PDiE, PE and PT in combination with cholesterol may be the ideal test lipids for crystallizing the native form of D2L and H1R receptors.
Conclusion
We have shown that the type of self-assembly lipid used influences reconstitution of functional D2L and H1R receptor. Our results indicate that an LCP with a small bilayer and water channel size is more suited to our native receptors compared to MO and this is irrespective of the receptor concentration. MO having the largest bilayer thickness and largest water channel size has proven to be one of the least favoured lipid for our receptors, whereas PE and PT having the smaller bilayer thickness and water channel size, were the most favoured lipids for reconstitution H1R and D2L, respectively.These results seem to indicate that the lipid bilayer thickness has a larger influence of the water channel diameter when reconstituting GPCRs and therefore a mismatch in LCP bilayer thickness seems to favour receptor reconstitution. The addition of cholesterol to MO was also found to improve D2L reconstitution and therefore serves as a viable additive for LCP reconstitution. In conclusion the LCP bilayer thickness should be considered when carrying out in meso crystallization trials and alternative lipid systems other than MO should be trialed which may reconstitute your GPCR more efficiently and improve the success rate of crystallization.Acknowledgements
We like to thank Dr. Charlotte Conn for supplying some of the self-assembly lipids for these results. The work was carried out in collaboration with the ARC Centre of Excellence in Advanced Molecular Imaging and partly funded by the CSIRO Preventative Health Flagship.References
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, et al. (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318: 1258-1265.
- White S (2015) Membrane proteins of known 3D structure. Structure 23: 1350-1361.
- Caffrey M (2008) On the mechanism of membrane protein crystallization in lipidic mesophases. Cryst Growth Des 8: 4244-4254.
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, et al. (2012) Structure of the human κ-opioid receptor in complex with JDTic. Nature 485: 327-332.
- Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, et al. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482: 552-556.
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, et al. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485: 321-326.
- Conn CE, Darmanin C, Sagnella SM, Mulet X, Greaves TL, et al. (2010) Incorporation of the dopamine D2L receptor and bacteriorhodopsin within bicontinuous cubic lipid phases. 1. Relevance to in meso crystallization of integral membrane proteins in monoolein systems. Soft Matter 6: 4828-4837.
- Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, et al. (2011) Structure of the human histamine H1 receptor complex with doxepin. Nature 475: 65-70.
- Chien EY, Liu W, Zhao Q, Katritch V, Han GW, et al. (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330: 1091-1095.
- Caffrey M, Cherezov V (2009) Crystallizing membrane proteins using lipidic mesophases. Nat Protoc 4: 706-731.
- Liang YL, Conn CE, Drummond CJ, Darmanin C (2015) Uptake of the butyrate receptors, GPR41 and GPR43, in lipidic bicontinuous cubic phases suitable for in meso crystallization. J Colloid Interface Sci 441: 78-84.
- Conn CE, Darmanin C, Sagnella SM, Mulet X, Greaves TL, et al. (2010) Incorporation of the dopamine D2L receptor and bacteriorhodopsin within bicontinuous cubic lipid phases. 2. Relevance to in meso crystallization of integral membrane proteins in novel lipid systems. Soft Matter 6: 4838-4846.
- Darmanin C, Conn CE, Newman J, Mulet X, Seabrook SA, et al. (2012) High-throughput production and structural characterization of libraries of self-assembly lipidic cubic phase materials. ACS Comb Sci 14: 247-252.
- Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Swillens S (1995) Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol Pharmacol 47: 1197-1203.