Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
*Address for Correspondence: Antonella Tosti MD, Department of Dermatology and Cutaneous Surgery, 1600 NW 10th Avenue, Rosenstiel Medical Science Building - Room 2023, Miami, FL 33136, USA, Tel: 305-243-8205; E-mail: ATosti@med.miami.edu
Citation: Lindsey SF, Tosti A. Hair Loss Induced by Tumor Necrosis Factor Alpha Inhibitors. J Clin Investigat Dermatol. 2013;1(1): 6.
Copyright © 2013 Tosti et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical & Investigative Dermatology | ISSN 2373-1044 | Volume: 1, Issue: 1
Submission: 18 October 2013 | Accepted: 20 November 2013 | Published: 25 November 2013
Reviewed & Approved by: Claudia I. Vidal, Assistant Professor, Department of Dermatology and a board certified pathologist and dermatopathologist, Saint Louis University, USA
The clinical presentation of alopecia areata occurring during anti-TNF-α therapy can vary widely ranging from mild scalp patches to alopecia areata universalis [21]. The most common presentation is patchy alopecia involving the parietal and occipital regions [4]. Etanercept-induced alopecia areata appears to be less severe, as patients mostly exhibit solitary patches of hair loss. Alopecia areata occurring during treatment with adalimumab and infliximab has a more varied clinical presentation ranging from patchy hair loss to alopecia areata universalis and totalis, respectively (Table 1).
Alopecia occurred between eight and 11 months after the initiation of therapy and varied widely. Two of the patients had focal scalp involvement with alopecia occurring as a patch in the frontal region [49] and along the temporal region and eyebrows [50], respectively. The third patient demonstrated symptoms diffusely throughout the temporal and occipital region of the scalp [51]. The ages ranged from eight to 56 years.
Hair Loss Induced by Tumor Necrosis Factor Alpha Inhibitors
Scott F. Lindsey1 and Antonella Tosti1,2*
- 1Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine Miami, USA
- 2Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, USA
*Address for Correspondence: Antonella Tosti MD, Department of Dermatology and Cutaneous Surgery, 1600 NW 10th Avenue, Rosenstiel Medical Science Building - Room 2023, Miami, FL 33136, USA, Tel: 305-243-8205; E-mail: ATosti@med.miami.edu
Citation: Lindsey SF, Tosti A. Hair Loss Induced by Tumor Necrosis Factor Alpha Inhibitors. J Clin Investigat Dermatol. 2013;1(1): 6.
Copyright © 2013 Tosti et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical & Investigative Dermatology | ISSN 2373-1044 | Volume: 1, Issue: 1
Submission: 18 October 2013 | Accepted: 20 November 2013 | Published: 25 November 2013
Reviewed & Approved by: Claudia I. Vidal, Assistant Professor, Department of Dermatology and a board certified pathologist and dermatopathologist, Saint Louis University, USA
Abstract
Background: Alopecia is a possible adverse reaction to Tumor Necrosis Factor alpha (TNF-α) inhibitors. This side effect has become more recognized in recent years through FDA postmarketing surveillance and it description in case reports/case series.Objective: We review the literature and summarize the pathogenesis, clinical presentation, prognosis, and management strategies for TNF-α inhibitor induced alopecia.
Methods: We performed a Medline search from January 1998 until August 2013 to identify all cases of alopecia during anti-TNF-α therapy described in the literature. We also reviewed FDA postmarketing data and clinical trials.
Results: There were 62 cases of hair loss occurring during therapy with TNF-α inhibitors that we identified during our literature search. The causes of hair loss included alopecia areata, psoriatic alopecia, lichen planopilaris, drug-induced lupus erythematosus, androgenetic alopecia, and telogen effluvium. Alopecia was also a mentioned side effect in three clinical trials and in FDA postmarketing surveillance.
Limitations: There are few controlled trials directly studying TNF-α inhibitor induced alopecia and most of our understanding of this clinical condition comes from anecdotal experience.
Conclusions: TNF-α inhibitors can cause different types of hair loss including severe alopecia areata and scarring alopecia.
Keywords
Tumor necrosis factor alpha inhibitors; Etanercept; Adalimumab; Infliximab; Alopecia; Alopecia areata; Psoriatic alopecia; Lichen planopilaris; Adverse reactionAbbreviations
TNF-α - Tumor Necrosis Factor Alpha; IL - Interleukin; IFN - Interferon; pDc - Plasmacytoid Predendritic Cells; LPP -Lichen Planopilaris; NSAID -Nonsteroidal Anti-Inflammatory Drug; DILE - Drug-Induced Lupus ErythematosusIntroduction
Tumor necrosis factor alpha (TNF-α) inhibitors comprise a specific class of biologic drugs that has become increasingly common, especially in dermatology. This class of medication is currently FDA approved for the treatment of Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, and juvenile idiopathic arthritis [1-3]. Through their off-label use, they have been shown to be effective for the treatment of a wide range of other immune-mediated conditions as well [4].There are three different tumor necrosis factor alpha inhibitors currently on the market: adalimumab, infliximab, and etanercept [1-3]. Adalimumab is a fully monoclonal human antibody [2] while infliximab is a chimeric mouse-human antibody [3]. These act by binding directly to both receptor-bound and freely circulating TNF-α molecules and neutralizing them [2,3]. Etanercept, on the other hand, is a TNF receptor-IgG fusion protein [1] and unlike adalimumab and infliximab, is unable to neutralize receptor bound TNF-α [5]. For this reason, adalimumab and infliximab are significantly more likely to induce apoptosis and cell lysis in inflammatory cells [5].
Because of their ability to accurately act on specific targets in the body, biologic drugs such as TNF-α inhibitors were thought to have fewer side effects than other traditional systemic therapies such as methotrexate and cyclosporine. However, as their use has become more widespread, new side effects of TNF-α antagonists have been reported [6-8]. One side effect of TNF-α inhibitors is alopecia. This has become more evident in recent years through publications in the literature and FDA postmarketing surveillance. One study reported that 3.3% of patients taking TNF-α inhibitors experience hair loss, and this can be an important reason for discontinuing therapy [9]. In this review, we will discuss the topic of TNF-α inhibitor induced alopecia and summarize the pathogenesis, clinical presentation, prognosis, and management strategies for the different causes of this condition.
Materials and Methods
A Medline search from January 1998 until August 2013 was performed to identify the cases described in the literature of hair loss occurring during anti TNF-α therapy. The terms used in Medline were (alopecia OR alopecia areata OR psoriasis) AND (infliximab OR etanercept OR adalimumab OR anti-TNF-alpha). We included case reports, case series, review articles, and clinical trials which specifically mentioned hair loss occurring during treatment with TNF-α inhibitors. We included cases of alopecia which occurred in the treatment of diseases during both FDA approved and the off-label use of TNF-α antagonists. We also reviewed the drug package inserts and the FDA postmarketing data.Results
Alopecia in Clinical trialsAlthough alopecia is an observed complication of anti TNF-α therapy, it is rarely studied directly in clinical trials and its true prevalence is therefore difficult to estimate. In two clinical trials of etanercept used to treat rheumatoid arthritis, alopecia was observed in six of 415 patients (1.45%) and one of 349 patients (0.29%) [1,10]. For infliximab, one study in patients with Crohn’s disease reported that one of 100 (1.00%) patients developed alopecia [11]. The authors were unable to find clinical trials directly measuring alopecia during treatment with adalimumab.
Alopecia in Postmarketing studies
Alopecia was found to be a relatively common side effect of anti TNF-α therapy in postmarketing studies [10,12]. It is listed as a potential adverse reaction in the package insert of Enbrel (etanercept) [1], Humira (adalimumab) [2], and Remicade (infliximab)[3]. Using eHealthMe to analyze data reported to the FDA [6-8], we attempted to estimate the prevalence of alopecia occurring with each TNF-α inhibitor. Alopecia was reported in 423 of the 79,722 (0.53%) people who reported side effects with infliximab [7], 1,495 of 166,330 (0.90%) patients with etanercept [6], and 1,291 of 130,505 (0.99%) patients with adalimumab [8]. It is important to note that this is self-reported information and these patients could be taking other agents which contribute to hair loss.
Alopecia in Case Reports/Case Series
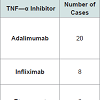
In the literature, we discovered 62 cases of hair loss occurring during therapy with TNF-α inhibitors. The causes of hair loss included alopecia areata, psoriatic alopecia, lichen planopilaris, drug-induced lupus erythematosus, androgenetic alopecia, and telogen effluvium. The patients who developed anti TNF-α induced alopecia were being treated for a wide range of conditions which included psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, Behçet’s disease, ankylosing spondylitis, rheumatoid arthritis, and idiopathic juvenile arthritis. There were 32 cases of alopecia described during treatment with adalimumab, 22 with infliximab, and nine with etanercept (in one case, both adalimumab and infliximab were used). By gender, there were 34 females, 25 males, and three unreported.
Clinical Subtypes of Alopecia Seen during Treatment with TNF-α InhibitorsAlopecia Areata:
Alopecia areata is a type of autoimmuneinduced non-scarring alopecia with a calculated lifetime risk of 2% [13]. The pathogenesis is not fully elucidated but studies suggest proinflammatory cytokines such as IL-1α, IL-1β, and TNF-α play a major role in disease progression [14]. As such, one would expect TNF-α inhibitors to be effective treatment options for this condition. Not only have studies shown this not to be the case [15], alopecia areata has actually been found to occur during treatment with TNF-α inhibitors. The first case was reported in 2004 with the use of infliximab [16] and since then we have identified 34 cases in the literature, including the TNF-α inhibitors adalimumab [4,17-27], infliximab [16,23,28-32], and etanercept (Table 1) [4,22,33,34]. In these cases, alopecia areata was diagnosed by both clinical presentation and histopathology, which was available in the majority of cases.
The clinical presentation of alopecia areata occurring during anti-TNF-α therapy can vary widely ranging from mild scalp patches to alopecia areata universalis [21]. The most common presentation is patchy alopecia involving the parietal and occipital regions [4]. Etanercept-induced alopecia areata appears to be less severe, as patients mostly exhibit solitary patches of hair loss. Alopecia areata occurring during treatment with adalimumab and infliximab has a more varied clinical presentation ranging from patchy hair loss to alopecia areata universalis and totalis, respectively (Table 1).
The average onset of symptoms was 9.9 months after the initiation of therapy, although presentation was documented to occur between 1 month and 3.5 years [4]. There were 15 males, 17 females, and two patients with unreported genders. The patients were being treated with TNF-α inhibitors for a wide range of conditions including psoriasis, psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, Crohn’s disease, and idiopathic juvenile arthritis. Most patients had no family or personal history of alopecia areata, although many suffered from other autoimmune conditions [4]. Interestingly, the occurrence of alopecia was noted to occur concurrently with other immune-mediated phenomena such as halo nevi [29] and psoriasiform eruptions [34].
Patients with alopecia areata occurring during anti TNF-α therapy have a mixed prognosis, with approximately one third achieving complete hair regrowth while others continue to progress to alopecia areata universalis and totalis. All patients with alopecia areata occurring during treatment with etanercept showed improvement in symptoms, although none of these patients achieved full hair regrowth. Patients with alopecia areata development during treatment with adalimumab and infliximab had a more varied prognosis, ranging from full hair regrowth to progression to alopecia areata totalis
There have been no clinical trials investigating the optimal treatment strategies for alopecia areata occurring during anti- TNF-therapy and most of our knowledge comes from anecdotal experience. The patients who achieved complete hair regrowth often did so after cessation of anti-TNF-α therapy although a minority of patients remained on the TNF-α inhibitor and still experienced some hair regrowth [4,35]. There are currently no accepted guidelines governing whether the TNF-α antagonist should be discontinued in these situations, and this should be evaluated on a case-by-case basis. The application of topical steroids to the scalp was initiated in almost all patients, although it is unclear whether this provided an actual benefit. Nakagomi et al. described a patient who achieved hair regrowth only after the initiation of cyclosporine [28]. Methotrexate was also initiated in some cases, usually with only minimal improvement [4].
The incidence of alopecia areata during anti-TNF-α therapy is unknown as there are no prospective controlled studies. Another open question is whether alopecia areata is actually induced by the medication, or whether this is simply a causal relationship which indicates that anti TNF-α therapy is not effective in preventing development of alopecia areata in predisposed individuals. Since TNF-α inhibitors are used for the treatment of autoimmune diseases, this patient population is drastically more susceptible to the development of other immune mediated conditions, including alopecia areata. These questions must be further explored.
Psoriatic Alopecia: The use of TNF-α inhibitors has become a staple of psoriasis treatment. This comes as no surprise, as TNF-α has been shown to be pivotal in the development of psoriasis [36]. Interestingly, TNF-α inhibitors also have a paradoxical effect of inducing psoriasis in between 1.5%-5% of patients [37,38]. This is thought to occur due to the complex interaction between TNF-α and interferon (IFN)α [39,40]. Plasmacytoid predendritic cells (pDCs) produce IFN-α, a pro-inflammatory cytokine that has been shown to cause psoriasis [41,42]. TNF-α acts on the pDCs and causes a decrease in the production and release of IFN-α [39]. It is hypothesized that through their indirect action in increasing IFN-α, TNF-α inhibitors have the ability to induce psoriasis.
Interestingly, the majority of these patients do not have a personal or family history of psoriasis.In our review of the literature, we found 19 cases of TNF-α inhibitor induced psoriasis leading to alopecia (Table 2). Interestingly, this was reported with infliximab and adalimumab but not etanercept.Symptoms ranged from patchy alopecia to total scalp hair loss. In addition to the scalp, these patients exhibited psoriatic lesions on the trunk, axillae, extremities, genitals, and palms/soles. The onset of hair loss ranged from two [43,44] to 46 [45] months after the TNF-α inhibitor was begun, with an average latency period of 8.6 months. There were 11 females and eight males. The patients were receiving anti TNF-α therapy for Crohn’s disease, ulcerative colitis, ankylosing spondylitis, juvenile idiopathic arthritis, and Behçet’s disease.
Patients with TNF-α inhibitor induced psoriatic alopecia had a generally positive prognosis, as greater than 75% achieved scalplesion clearance and complete hair regrowth after the medication was discontinued. Virtually all of the patients had some improvement in symptoms. One patient continued to exhibit diffuse patchy alopecia after adalimumab was discontinued, and hair loss was only halted after the initiation of cyclosporine 3mg/kg/day [46]. Topical steroids were applied in the majority of the cases although they appeared to have little positive effect without concomitant discontinuation of the TNF-α inhibitor. In three cases, the medication was continued and improvement was still appreciated with topical agents. Scarring alopecia, documented by pathology, occurred in 16% of the cases (three of 19 patients). Scarring alopecia is a possible evolution of severe scalp psoriasis which has been linked to severe inflammation and sebaceous gland destruction [47].
There are multiple cases in the literature describing psoriasiform eruptions of the skin occurring in parallel with alopecia areata [28]. This presents the clinical challenge of differentiating TNF-α induced psoriatic alopecia and TNF-α induced alopecia areata. Doyle et al. proposed that this may in fact represent a distinct entity referred to as “psoriatic alopecia/alopecia areata-like reaction secondary to anti- TNF treatment.” [35] The authors suggest that this can be diagnosed by histopathology where the presence of plasma cells and eosinophils can be used to distinguish “anti-TNF induced alopecia” from psoriatic alopecia and alopecia areata. It remains unclear whether this in fact represents a distinct clinical entity or rather a subset of the aforementioned diseases.
Lichen Planopilaris:
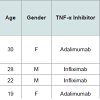
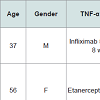
Lichenoid drug reactions are a wellestablished adverse reaction to TNF-α inhibitors [48]. A rare but reported subcategory includes TNF-α inhibitor induced lichen planopilaris (LPP). LPP represents an immune-mediated inflammatory disorder which causes a scarring alopecia of the follicular apparatus [49]. There have been three reported cases to date of LPP occurring during treatment with a TNF-α inhibitor (Table 3). The diagnosis was confirmed in each case by histopathology. The patients were being treated for psoriasis and psoriatic arthritis.
Alopecia occurred between eight and 11 months after the initiation of therapy and varied widely. Two of the patients had focal scalp involvement with alopecia occurring as a patch in the frontal region [49] and along the temporal region and eyebrows [50], respectively. The third patient demonstrated symptoms diffusely throughout the temporal and occipital region of the scalp [51]. The ages ranged from eight to 56 years.
These patients had a relatively poor prognosis. In two cases, the TNF blocking agent was continued because of the patients’ positive response to a previously refractory psoriasis. The lesions failed to improve after the addition of topical steroids and topical tacrolimus [49] and oral deflazacort [50], respectively. The third patient was initially taken off therapy with etanercept and the lesions disappeared with cyclosporine, NSAIDs, and topical steroids [51]. After a recurrence of severe psoriatic arthritis, he was restarted on etanercept and the LPP recurred with new areas of involvement. Etanercept was permanently stopped but the alopecia did not improve with methotrexate and NSAIDs.
Drug-Induced Lupus Erythematosus:
Drug-induced lupus erythematosus (DILE) is an established complication of TNF-α inhibitors [52]. Classic symptoms include myalgia, arthralgia, rash and serositis in conjunction with abnormal laboratory values [52]. Rarely, alopecia can occur [52-55]. Carlson et al. described a 48-yearold woman who developed DILE after two years of treatment with etanercept for rheumatoid arthritis diagnosed after she presented with a two week history of diffuse hair loss, skin lesions, and abnormal laboratory values [55]. After discontinuing etanercept and receiving prednisone, her symptoms improved. After seven months, she had complete hair regrowth and her laboratory values normalized. In a French national study, de Bandt also described a patient who developed alopecia as a symptom of DILE [53]. The patient was being treated for erosive rheumatoid arthritis. The specific TNF-α inhibitor used was not disclosed.
Androgenetic Alopecia/Telogen Effluvium: In rare cases, TNF-α antagonists have been found to precipitate androgenetic alopecia. Lee et al. described a 39-year-old woman who developed androgenetic alopecia after being treated with adalimumab for rheumatoid arthritis [26]. The patient also exhibited telogen effluvium [26].
Conclusion
TNF-α antagonists are an exciting new class of medication used for the treatment of many autoimmune diseases previously refractory to conventional therapies. As their use is becoming more prevalent, their side effects are becoming better documented. One little-known complication that has become more evident in recent years is alopecia.TNF-α inhibitor induced alopecia is especially difficult for the physician to manage because there are no well-defined treatment guidelines and many treatment strategies come from anecdotal experience. Although there are many reports of its occurrence in the literature and in postmarketing surveillance, there are few clinical trials where it is directly studied and its prevalence is therefore hard to estimate. It is also difficult to determine whether certain anti TNF agents are more likely to cause alopecia than others.
When the physician encounters new-onset alopecia in a patient on anti TNF-α therapy, the risk/benefit ratio must be weighed before any change in therapy is considered. TNF-α inhibitors have the ability toinduce disease remissions in patients with debilitating autoimmune conditions refractive to traditional therapies. In some patients the benefit from treatment may outweigh the risk of permanent hairloss. For this reason, it is important for the physician to handle each situation case-by-case, taking the patient’s priorities into consideration. As a general rule, we recommend switching to another medication in patients with alopecia areata or psoriatic alopecia occurring during anti TNF-α therapy. This can include another TNF-α inhibitor, or a different class of immunomodulating drug such as methotrexate or cyclosporine, depending on the condition being treated. We also recommend the application of topical steroids under occlusion.
TNF- α blockers are a very useful class of medication that have the ability to drastically improved the lives of patients. As such, the side effect of alopecia may be considered by some to be relatively inconsequential compared to the debilitating disease being treated. The purpose of this paper is not to discourage the use of anti TNF-α therapy but to inform physicians about this potential side effect so that they may be more able to manage it if encountered in their practice.
References
- Enbril (Etanercept) [package Insert]. Thousand Oaks, CA: Immunex Corp; 2003.
- Humira (Adalimumab) [package Insert]. Wellington, New Zealand: AbbVie Inc; 2013.
- Remicade (Infliximab) [package Insert]. Einsteinweg, The Netherlands: Janssen Biologics; 2009.
- Ferran M, Calvet J, Almirall M, Pujol RM, Maymo J (2011) Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-alpha blocker agents: Report of five cases and review of the literature. J Eur Acad Dermatol Venereol 25: 479-484.
- Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, et al. (2008) Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum 58: 1248-1257.
- From FDA Reports: Enbrel and Alopecia. eHealthMe [Internet]. 2013 August [cited 2013 Aug 24].
- FDA Reports: Remicade and Alopecia. eHealthMe [Internet]. 2013 August [cited 2013 Aug 24].
- From FDA Reports: Humira and Alopecia. eHealthMe [Internet]. 2013 August [cited 2013 Aug 24].
- Lutf A, Hammoudeh M (2012) Weight Gain and Hair Loss during Anti-TNF Therapy. Int J Rheumatol 2012: 593039.
- Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, et al. (2006) Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 10: 1-239.
- Farrell RJ, Shah SA, Lodhavia PJ, et al. (2000) Clinical experience with infliximab therapy in 100 patients with Crohn's disease. Am J Gastroenterol 95: 3490-3497.
- UPDATE ON THE TNF-ALPHA BLOCKING AGENTS. FDA [Internet]. 2002 [cited 2013 Aug 24].
- Gilhar A, Etzioni A, Paus R (2012) Alopecia areata. N Engl J Med 366: 1515-1525.
- Philpott MP, Sanders DA, Bowen J, Kealey T (1996) Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol 135: 942-948.
- Strober BE, Siu K, Alexis AF, et al. (2005) Etanercept does not effectively treat moderate to severe alopecia areata: an open-label study. J Am Acad Dermatol 52: 1082-1084.
- Ettefagh L, Nedorost S, Mirmirani P (2004) Alopecia areata in a patient using infliximab: new insights into the role of tumor necrosis factor on human hair follicles. Arch Dermatol 140: 1012.
- Katoulis AC, Alevizou A, Bozi E, Georgala S, Mistidou M, et al. (2009) Biologic agents and alopecia areata. Dermatology 218: 184-185.
- Chaves Y, Duarte G, Ben-Said B, Tebib J, Berard F, et al. (2008) Alopecia areata universalis during treatment of rheumatoid arthritis with anti-TNF-alpha antibody (adalimumab). Dermatology 217: 380.
- Pelivani N, Hassan AS, Braathen LR, Hunger RE, Yawalkar N. (2008) Alopecia areata universalis elicited during treatment with adalimumab. Dermatology 216: 320-323.
- Kirshen C, Kanigsberg N (2009) Alopecia areata following adalimumab. J Cutan Med Surg 13: 48-50.
- Garcia Bartels N, Lee HH, Worm M, Burmester GR, Sterry W, et al. (2006) Development of alopecia areata universalis in a patient receiving adalimumab. Arch Dermatol 142: 1654-1655.
- Le Bidre E, Chaby G, Martin L, Perrussel M, Sassolas B, et al. (2011) [Alopecia areata during anti-TNF alpha therapy: Nine cases]. Ann Dermatol Venereol 138: 285-293.
- 23. Exarchou SA, Voulgari PV, Markatseli TE, Zioga A, Drosos AA (2009) Immune-mediated skin lesions in patients treated with anti-tumour necrosis factor alpha inhibitors. Scand J Rheumatol 38: 328-331.
- Navarro R, Dauden E, Gallo E, Santiago Sanchez-Mateos D, Garcia-Diez A (2012) Alopecia areata during treatment of psoriasis with adalimumab and leflunomide: a case and review of the literature. Skin Pharmacol Physiol 25: 107-110.
- Neila J, Carrizosa A, Ceballos C, Camacho FM. (2011) [Alopecia areata after biologic therapy: report of a case related to adalimumab]. Actas Dermosifiliogr 102: 827-828.
- Lee HH, Song IH, Friedrich M, Gauliard A, Detert J, et al. (2007) Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol 156: 486-491.
- Zschoche C, Bidier M, Hadaschik E. (2013) Alopecia areata during treatment with adalimumab: therapy with an alternative TNF-alpha inhibitor is possible. Journal der Deutschen Dermatologischen Gesellschaft = J Dtsch Dermatol Ges 11: 450-453.
- Nakagomi D, Harada K, Yagasaki A, Kawamura T, Shibagaki N, et al. (2009) Psoriasiform eruption associated with alopecia areata during infliximab therapy. Clin Exp Dermatol 34: 923-924.
- Fabre C, Dereure O (2008) Worsening alopecia areata and de novo occurrence of multiple halo nevi in a patient receiving infliximab. Dermatology 216: 185-186.
- Tosti A, Pazzaglia M, Starace M, Bellavista S, Vincenzi C, et al. (2006) Alopecia areata during treatment with biologic agents. Arch Dermatol 142: 1653-1654.
- Beccastrini E, Squatrito D, Emmi G, Fabbri P, Emmi L. (2010) Alopecia areata universalis during off-label treatment with Infliximab in a patient with Behcet disease. Dermatol Online J 16: 15.
- Hernandez MV, Nogues S, Ruiz-Esquide V, Alsina M, Canete JD, et al. (2009) Development of alopecia areata after biological therapy with TNF-alpha Blockers: description of a case and review of the literature. Clin Exp Rheumatol 27: 892-893.
- Posten W, Swan J (2005) Recurrence of alopecia areata in a patient receiving etanercept injections. Arch Dermatol 141: 759-760.
- Pan Y, Rao NA (2009) Alopecia areata during etanercept therapy. Ocul Immunol Inflamm 17: 127-129.
- Doyle LA, Sperling LC, Baksh S, Lackey J, Thomas B, et al. (2011) Psoriatic alopecia/alopecia areata-like reactions secondary to anti-tumor necrosis factor-alpha therapy: a novel cause of noncicatricial alopecia. Am J Dermatopathol 33: 161-166.
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, et al. (2004) Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med 199: 731-736.
- Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. (2005) Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum 52: 2513-2518.
- Medkour F, Babai S, Chanteloup E, Buffard V, Delchier JC, et al. (2010) Development of diffuse psoriasis with alopecia during treatment of Crohn's disease with infliximab. Gastroenterol Clin Biol 34: 140-141.
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J (2005) Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A 102: 3372-3377.
- Manni E, Barachini P (2009) Psoriasis induced by infliximab in a patient suffering from Crohn's disease. Int J Immunopathol Pharmacol 22: 841-844.
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, et al. (2005) Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 202: 135-143.
- Ettler J, Wetter DA, Pittelkow MR (2012) Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-alpha inhibitor therapy. Clinical Clin Exp Dermatol 37: 639-641.
- El Shabrawi-Caelen L, La Placa M, Vincenzi C, Haidn T, Muellegger R, et al. (2010) Adalimumab-induced psoriasis of the scalp with diffuse alopecia: a severe potentially irreversible cutaneous side effect of TNF-alpha blockers. Inflamm Bowel Dis 16: 182-183.
- Perman MJ, Lovell DJ, Denson LA, Farrell MK, Lucky AW (2012) Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol 29: 454-459.
- Osorio F, Magro F, Lisboa C, Lopes S, Macedo G, et al. (2012) Anti-TNF-alpha induced psoriasiform eruptions with severe scalp involvement and alopecia: report of five cases and review of the literature. Dermatology 225: 163-167.
- Papadavid E, Gazi S, Dalamaga M, Stavrianeas N, Ntelis V (2008) Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: a case series of four patients and guidelines for management. J Eur Acad Dermatol Venereol 22: 380-382.
- Silva CY, Brown KL, Kurban AK, Mahalingam M (2012) Psoriatic alopecia - fact or fiction? A clinicohistopathologic reappraisal. J Am Acad Dermatol 78: 611-619.
- Asarch A, Gottlieb AB, Lee J, Masterpol KS, Scheinman PL, et al. (2009) Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol 61: 104-111.
- Abbasi NR, Orlow SJ (2009) Lichen planopilaris noted during etanercept therapy in a child with severe psoriasis. Pediatr Dermatol 26: 118.
- Fernandez-Torres R, Paradela S, Valbuena L, Fonseca E (2010) Infliximab-induced lichen planopilaris. Ann Pharmacother 44: 1501-1503.
- Garcovich S, Manco S, Zampetti A, Amerio P, Garcovich A (2008) Onset of lichen planopilaris during treatment with etanercept. Br J Dermatol 158: 1161-1163.
- Costa MF, Said NR, Zimmermann B (2008) Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum 37: 381-387.
- De Bandt M, Sibilia J, Le Loet X, Prouzeau S, Fautrel B, et al. (2005) Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 7: R545-551.
- Ramos-Casals M, Brito-Zeron P, Soto MJ, Cuadrado MJ, Khamashta MA (2008) Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol 22: 847-861.
- Carlson E, Rothfield N (2003) Etanercept-induced lupus-like syndrome in a patient with rheumatoid arthritis. Arthritis Rheum 48: 1165-1166.