Journal of Cardiobiology
Download PDF
Research Article
*Address for Correspondence: Aurelio Leone, MD, FASH, FRSPH, Independent Researcher and Former Director, Department of Internal Medicine City Hospital, Via Provinciale 27, 19030 Castelnuovo Magra (SP), Massa, Italy, Tel: +393472272215; E-mail: reliol@libero.it
Citation: Leone A. Relation between Coronary Lesions and Cigarette Smoking of Subjects Deceased from Acute Myocardial Infarction. A Histopathological Study. J Cardiobiol. 2014;2(2): 5.
Copyright © 2014 Leone A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 2, Issue: 2
Submission: 22 February, 2014 | Accepted: 13 March, 2014 | Published: 17 March, 2014
Reviewed & Approved by: Dr. Luanda Grazette, Advanced Heart Failure and Cardiomyopathy, Keck School of Medicine, University of Southern California, USA
Relation between Coronary Lesions and Cigarette Smoking of Subjects Deceased from Acute Myocardial Infarction. A Histopathological Study
Aurelio Leone*
- Department of Internal Medicine City Hospital, Massa, Italy
*Address for Correspondence: Aurelio Leone, MD, FASH, FRSPH, Independent Researcher and Former Director, Department of Internal Medicine City Hospital, Via Provinciale 27, 19030 Castelnuovo Magra (SP), Massa, Italy, Tel: +393472272215; E-mail: reliol@libero.it
Citation: Leone A. Relation between Coronary Lesions and Cigarette Smoking of Subjects Deceased from Acute Myocardial Infarction. A Histopathological Study. J Cardiobiol. 2014;2(2): 5.
Copyright © 2014 Leone A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 2, Issue: 2
Submission: 22 February, 2014 | Accepted: 13 March, 2014 | Published: 17 March, 2014
Reviewed & Approved by: Dr. Luanda Grazette, Advanced Heart Failure and Cardiomyopathy, Keck School of Medicine, University of Southern California, USA
Abstract
Post-mortem studies on the relationship between coronary artery lesions and cigarette smoking in smoker subjects who died from AMI are numerically of scarce consistency if compared to the epidemiological and clinical findings. However, autopsy examination provides certain results on the definition of the existing link.This study, which analyzed 80 autopsy cases of smokers (n° 68) and non-smokers (n° 12) as a control group who died from AMI, showed that coronary arteries of smokers displayed more severe narrowing partially or totally occluding vessel lumen because of a superimposed thrombus and a higher incidence of three vessel coronary disease with a statistically significant difference. Calcium deposits in the coronary wall were also seen. However, the type and morphology of the alterations in both groups were similar to that observed in the lesions that usually can be documented in the histologic specimens of patients who died from AMI, any cause determined.
Keywords
Myocardial infarction; Coronary artery pathology; Cigarette smokingIntroduction
Cigarette smoking has been stably recognized as a major cardiovascular risk factor by the American Heart Association since evidence indicates that both types, active and passive smoking, highly increase the rate of coronary artery disease [1-5].A growing evidence, particularly of some reports [6-15], contribute to identify mainly the clinical, epidemiologic and functional characteristics of the cardiovascular effects related to smoking, while less attention has been given to the morphology of pathological lesions, which occur as a consequence of smoking compound activity.
Nicotine and carbon monoxide exert their harmful effects on the heart and blood vessels, although the development of the lesions depends on both the place where alterations appear and duration of smoking exposure [16,17]. Evidence indicates that functional disorders are prevailingly associated with the activity of nicotine and its metabolites, whereas morphological alterations are the results of chronic and prolonged exposure to smoking and are mostly related to the toxic effect of carbon monoxide.
The purpose of this study was to retrospectively analyze the main morphological coronary lesions in smoker subjects deceased from acute myocardial infarction (AMI) undergone post-mortem exam.
Materials and Methods
80 subjects, 62 men (77.5%) and 18 females (22.5%) aged from 48 to 78 years (mean: 65.8+/-13.4 years) died from AMI and underwent the post-mortem study. Sixty-eight subjects (85%) were smokers, while 12 non-smokers (15%) were as a control group. Table 1 summarizes the main characteristics of the study population.
Post-mortem examination of the heart and coronary vessels was carried out by using a method previously described [18-21] (Figures 1 and 2).
In each patient, the heart was removed severing the pulmonary artery and aorta about 5 cm above the free margin of the semilunar valves. After observing and recording the external aspect of the heart to identify macroscopically the areas of myocardial lesions, without cutting the heart and coronary vessels, a rubber was placed through the aorta into the aortic orifice to impede the flux of liquid material to the left ventricular cavity and, then, the coronary arteries were injected at a pressure of 130 mmHg using a barium-iodine-gelatin radiopaque mass by a canoe tied into the aorta (Figure 1). When a good degree of contrast was observed by means of x-rays, all the hearts were fixed in 10% water-formalin solution. Then, five to six transverse slices of thickness approximately 1 cm were cut from the apex to the base (Figure 2). These slices were parallel to each other and atrioventricular sulcus. The thickness of the ventricular wall of each slice was measured and its mean mathematically measured. In addition, the location, size, and age of myocardial alterations, specifically infarct, were recorded and percent extent quantified in comparison to the total myocardium by using a polar planimeter and, then, photographed.
Post-mortem examination of the heart and coronary vessels was carried out by using a method previously described [18-21] (Figures 1 and 2).
The coronary vessels were examined cutting each major coronary artery and its branches transversely along the entire length at an interval of an approximately half centimeter. All observed alterations were measured according to their length and degree and recorded.
Microscopic examination of both myocardial slices and coronary artery segments was carried out after hematoxylin-eosin staining as described by Mallory et al. [22].
Every removed segment of the coronary arteries together with intramyocardial vessels was analyzed. A total of 400 specimens were examined for both groups, smokers and the control group. Artery wall alterations, intraluminal narrowing, and thrombi partially or totally occluding the vessel lumen recorded. When partial narrowing affected the internal diameter of the coronary vessel, its degree was measured and calculated in percent as compared to the normal internal diameter with an ocular micrometer. Therefore, study the degree and length of the narrowing were carefully established. Accordingly to a previous study [20], four types of narrowing were classified: 1 to 30%, 31 to 60%, 61 to 90%, and over 90%. A narrowing over 90% was considered as practically occluding the coronary lumen.Table 2 summarizes the main characteristics of the methodological examination of the coronary arteries in the studied patients.
The coronary alterations observed in smokers and control group were statistically tested by using t-test with a statistically significant estimate of P < 0.05.
The coronary alterations observed in smokers and control group were statistically tested by using t-test with a statistically significant estimate of P < 0.05.
Results
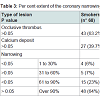
68 autopsy cases with AMI were smokers (85%), while the other 12 (15%) non-smokers act as a control group. Similar lesions characterized both smokers and non-smokers with regard to occlusive thrombi (respectively 43-63.2% and 5-41.5%, P>0.05) and calcium deposits in the artery wall (respectively 27-39.7% and 4-33.3%, P>0.05). These alterations did not show a statistically significant difference, although, numerically, they were prevailing in the smoker subjects.Different observations were related to the degree of narrowing and the number of coronary arteries affected.
The smoker group displayed a narrowing between 1 to 30% in 4 cases (6%), 31 to 60% in 5 (7%), 61 to 90 % in 15 (23%), and over 90% in 48, including the subjects showing occlusive thrombi. The control group displayed the following records: 1 to 30% in four cases (33%), 31 to 60 % in 2 (17%), 61 to 90% in 1 (8%), and over 90% in 5 cases (42%), also including subjects with an occluding thrombus. P<0.05 was observed for the narrowing between 1 to 30% to the advantage of the non-smoker group and between narrowing between 61 to 90% of the smoker subjects.
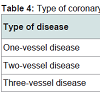
One, two, and three vessel coronary disease affected both groups, but with a statistically significant difference (P&l;0.05) for the smokers with regard to three vessel disease. One vessel disease affected 51 smokers (75%) vs 11 (91.6%) non-smokers, two vessel disease was demonstrated in 10 smokers (14.7%) vs 1 (8.4%) of non-smokers, and three vessel disease characterized 7 smokers (10.3%), but none nonsmoker.
Discussion
It is worth noting that a fundamental concept emerges from this study. Since it is a morpho-pathological study that analyzes autopsy material, the observed alterations are really existing and documented with no doubt. In addition, there is evidence of a greater extent and number of structural lesions in the coronary arteries of the smokers, who died from AMI, out of any statistical evaluation. Such evidence would confirm the deleterious effects due to smoking exposure at different levels of the cardiovascular system as reported by several papers [23-27].With regard to the type of structural alterations documented in the coronary arteries of this study, three factors play a strong role: the incidence and type of narrowing, calcium deposits, and number of arteries affected.
Coronary narrowing has to be considered the anatomical substrate of the alterations caused by cigarette smoking since it is related to luminal vessel patency and, therefore, to coronary blood flow. However, the degree and extent of the narrowing do not determine the characteristics of myocardial necrosis. Indeed, two types of necrosis are usually recognized as a consequence of cigarette smoking [28-30]: ischemic necrosis due to occluding pathology of coronary circulation and toxic necrosis as an effect of chemical substances able to damage the myocardium. Thus, cigarette smoking may cause both types of necrosis, which, therefore, occurs without coronary alterations in some cases. Myocardial necrosis is the most serious event that nicotine and carbon monoxide can cause [31-33].
Generally, the pathological lesions of the coronary arteries are prevailing because cigarette smoking triggers endothelial dysfunction, which has been documented to be the “door” of atherosclerosis [34-37] that is characterized by thrombotic alterations. However, the effects of necrosis of the myocardium are almost similar in both types.
Calcium deposits have been observed in the coronary arteries of both smokers and non-smokers with no statistically significant difference in this study.
Evidence indicates that deposits of calcium are a common observation in the atherosclerotic artery vessels [38]. Therefore, they may be the result of any factor able to cause coronary atherosclerosis including cigarette smoking.
Calcium deposits have been found in the arterial wall together with a major incidence and extent of thrombi [39] particularly in smokers. These observations also involve hemostatic parameter alterations primarily fibrinogen with documented disorders in blood fibrinogen concentrations. Therefore, on the one hand, increased amount in calcium deposits and on the other changes in coagulation-fibrinolysis cascade well explain the reasons of the greater incidence of thrombus in the coronary arteries of smokers died from AMI. However, these abnormalities are not a specific pattern of smokers because they may be common to similar ischemic pathology caused by other factors.
Morphologically, calcium deposits are usually seen in the context of atheromas in presence of advanced coronary atherosclerosis together with lipid accumulation as an effect of both nicotine and carbon monoxide [30].
Increased calcium deposits on vascular tree affect the arteries of active smokers and exposed never smokers to a significantly major rate when these two groups are compared with non-exposed never smokers [39]. However, similar conclusions are not reached by this study.
Usually, these anatomical changes have an unfavorable prognosis, particularly for those individuals suffering from pre-existing ischemic heart disease and undergone an infarct.
Initially, calcium deposits may be documented in the mitochondria of dead cells or cells which are dying and, then, fill irregularly the entire atheroma. Apart the major occurrence of thrombus, the most typical lesion determined by calcium deposits consists of an increased stiffness of the arterial wall with alterations of coronary blood flow as an effect of the reduced elasticity of the vessel.
The distribution and extent in the number of coronary arteries affected is worthy of a different mention when smokers died from AMI are compared with similar subjects, but non-smokers as the present study clearly shows.
Three vessel disease has been observed in the smokers who died from AMI with a statistically significant difference if compared to non-smokers. Indeed, evidence indicates that three vessel disease has deleterious effects on myocardial function and causes severe hemodynamic impairment [40]. Subjects with recurrent angina pectoris at rest often displayed three vessel coronary disease and left ventricular dysfunction. A higher prevalence of three vessel coronary disease was documented in smokers by studies of Sukhija et al. and Frid et al. who also demonstrated angiographically that smoking cessation produced a reduction in the extent and number of coronary alterations [41,42]. Therefore, both the number of coronary arteries involved and degree and number of narrowing of the vessel lumen undoubtedly influence the anatomical characteristics of the lesions in the smokers.
One and two vessel coronary artery disease have been observed in both smokers and non-smokers died from AMI with no statistical difference in this study.
It is worth noting that the pathology of the coronary arteries observed at the autopsy in individuals who died from AMI is characterized by multiple aspects going from no or minimal coronary alterations [43-45] up to the occurrence of severe involvement [46] mainly related to the features of the atherosclerotic plaque [47,48]. However, usually smokers display multi-vessel coronary disease and characteristic lesions consisting of multiple coronary narrowing and thrombi partially or totally occluding vessel lumen at post-mortem examination. However, no clear approach exists to establish the threshold to which a coronary vessel is related to ischemic heart disease. As a paper of Baroldi et al. undoubtedly shows, both the degree of vessel occlusion and its histopathological characteristics play a similar effect [19].
Conclusion
The results of this study, which has been conducted in patients who died from AMI, clearly show that a strong relationship between cigarette smoking and coronary artery lesions exists as the parameters analyzed at the autopsy undoubtedly have documented. In addition, this study underlines how post-mortem findings, which are often neglected to an advantage of clinical and epidemiological observations, provide certain results, although related to a small number of cases in comparison with, on the deleterious effects of cigarette smoking on coronary arteries.Finally, more severe coronary narrowing and multi-vessel coronary disease characterized the smokers who died from AMI and were the distinguish marker of cigarette smoking.
References
- Glantz SA, Parmley WW (1995) Passive smoking and heart disease. JAMA 273: 1047-1053.
- Leone A (1993) Cardiovascular damage from smoking: a fact or belief? Int J Cardiol 38: 113-117.
- Leone A (2003) Relationship between cigarette smoking and other coronary risk factors in atherosclerosis: risk of cardiovascular disease and preventive measures. Curr Pharm Des 9: 2417-2423.
- Wells AJ (1994) Passive smoking as a cause of heart disease. J Am CollCardiol 24: 546-554.
- Towler DA (2013) The “perfect storm” of cardiometabolic risk illuminates genetic diathesis in cardiovascular disease. J Am Coll Cardiol 62: 799-7801.
- Glantz SA, Parmley WW (1991) Passive smoking and heart disease: Epidemiology, physiology, and biochemistry. Circulation 83: 1-12.
- McBride PE (1992) The health consequences of smoking. Cardiovascular diseases. Med Clin North Am 76: 333-353.
- Reid DD, Hamilton PJ, McCartney P, Rose G, Jarrett RJ, et al. (1976) Smoking and other risk factors for coronary heart disease in British civil servants. Lancet 2: 979-984.
- Wald NJ, Hackshaw AK (1996) Cigarette smoking: an epidemiological overview. Br Med Bull 52: 3-11.
- Peto R, Lopez AD, Boreham J, Thun M, Heath C (1994) Mortality from smoking in developed countries: 1950-2000. Oxford University Press, Oxford.
- Doyle JT, Dawber TR, Kannel WB, Kinch SH, Kahn HA (1964) The relationship of cigarette smoking to coronary heart disease: The second report of the combined experience of the Albany, NY, and Framingham, Mass, studies. JAMA 190: 886-890.
- Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R (1999) Passive smoking as well as active smoking increase the risk of acute stroke. Tob Control 8: 156-160.
- Leone A, Bertanelli F, Mori L, Fabiano P, Bertoncini G (1992) Ventricular arrhythmias by passive smoke in patients with preexisting myocardial infarction. J Am Coll Cardiol 3: 256a.
- Burns DM (2003) Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 46: 11-29.
- Heidrich J, Wellmann J, Heuschmann PU, Kraywinkel K, Keil U (2007) Mortality and morbidity from coronary heart disease attributable to passive smoking. Eur Heart J 28: 2498-2502.
- Benowitz NL, Jacob P 3rd, Jones RT, Rosemberg J (1982) Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exper Ther 221: 368-372.
- Leone A (2012) How and why chemicals from tobacco smoke can induce a rise in blood pressure. World J Pharmacol 1: 10-20.
- Leone A (1972) L’angiografia coronarica post-mortem nello studio anatomo-patologico del cuore. G Ital Cardiol 2: 488-492
- Baroldi G, Radice F, Schmidt C, Leone A (1974) Morphology of acute myocardial infarction in relation to coronary thrombosis. Am Heart J 87: 65-75.
- Leone A, Fabiano P, Bertanelli F, Mori L, Bertoncini G (1992) Postinfarction cardiac rupture in the nineties: do we know determining factors? Singapore Med J 33: 282-286.
- Leone A (1991) Are we able to prevent death due to postinfarction cardiac rupture by early diagnosis and surgical treatment. Jpn Heart J 32: 635-644.
- Mallory GK, White PD, Salcedo-Salgar J (1939) The speed of healing of myocardial infarction. A study of the pathologic anatomy in seventy two cases. Am Heart J 18: 647-671.
- Sherman CB (1991) Health effects of cigarette smoking. Clin Chest Med 12: 643-658.
- Leone A (1995) Cigarette smoking and health of the heart. J Roy Soc Health 115: 354-355.
- Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, et al. (1993) Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am CollCardiol 22: 642-647.
- Zhu BQ, Parmley WW (1995) Hemodynamic and vascular effects of active and passive smoking. Am Heart J 130: 1270-1275.
- Klein LW, Pichard AD, Holt J, Smith H, Gorlin R, et al. (1983) Effects of chronic tobacco smoking on coronary circulation. J Am CollCardiol 1: 421-426.
- Astrup P, Kjeldsen K (1979) Model studies linking carbon monoxide and/or nicotine to arteriosclerosis and cardiovascular disease. Prev Med 8: 295-302.
- Bajusz E, Jasmin G (1965) Observations on histochemical differential-diagnosis between primary and secondary cardiomyopathies. Am Heart J 69: 83-92.
- Leone A, Landini L Jr, Biadi O, Balbarini A (2008) Smoking and cardiovascular system: cellular features of the damage. Curr Pharm Des 14: 1771-1777.
- Dwyer EM Jr, Turino GM (1989) Carbon monoxide and cardiovascular disease. N Engl J Med 321: 1474-1475.
- Leone A, Bertanelli F, Mori L, Fabiano P, Battaglia A (1994) Features of ischaemic cardiac pathology resulting from cigarette smoking. J Smoking-Related Dis 5: 109-114.
- Auerbach O, Carter HW, Garfinkel L, Hammond EC (1976) Cigarette smoking and coronary heart disease. A macroscopic and microscopic study. Chest 70: 697-705.
- Desideri G, Ferri C (2005) Endothelial activation. Sliding door to atherosclerosis. Curr Pharm Des 11: 2163-2175.
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, et al. (1993) Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149-2155.
- Penn A, Chen LC, Snyder CA (1994) Inhalation of steady-state sidestream smoke from one cigarette promotes arteriosclerotic plaque development. Circulation 90: 1363-1367.
- Leone A, Balbarini A (2008) Exposure to passive smoking: a test to predict endothelial dysfunction and atherosclerotic lesions. Angiology 59: 220-223.
- Schmermund A, Baumgart D, Gorge G, Seibel R, Grönemeyer D, et al. (1997) Coronary artery calcium in acute coronary syndromes: a comparative study of electron-beam computed tomography, coronary angiography, and intracoronary ultrasound in survivors of acute myocardial infarction and unstable angina.. Circulation 96: 1461-1469.
- Goel M, Wong ND, Eisenberg H, Hagar J, Kelly K, et al. (1992) Risk factor correlates of coronary calcium as evaluated by ultrafast computed tomography. Am J Cardiol 70: 977-980.
- Gorgels AP, Vos MA, Mulleneers R, de Zwaan C, Bär FW, et al. (1993) Value of the electrocardiogram in diagnosing the number of severely narrowed coronary arteries in rest angina pectoris. Am J Cardiol 72: 999-1003.
- Sukhija R, Yalamanchili K, Aronow WS (2003) Prevalence of left main coronary artery disease, of three-or four-vessel coronary artery disease, and of obstructive coronary artery disease in patients with and without peripheral arterial disease undergoing coronary angiography for suspected coronary artery disease. Am J Cardiol 92: 304-305.
- Frid D, Ockene IS, Ockene JK, Merriam P, Goldberg R, et al. (1991) Severity of angiographically proven coronary artery disease predicts smoking cessation. Am J Prev Med 7: 131-135.
- Eliot RS, Baroldi G, Leone A (1974) Necropsy studies in myocardial infarction with minimal or no coronary luminal reduction due to atherosclerosis. Circulation 49: 1127-1131.
- Roberts WC (1972) Coronary arteries in fatal acute myocardial infarction. Circulation 45: 215-230.
- Marius-Nunez AL (1990) Myocardial infarction with normal coronary arteries after acute exposure to carbon monoxide. Chest 97: 491-494.
- Chandler AB, Chapman I, Erhardt LR, Roberts WC, Schwartz CJ, et al. (1974) Coronary thrombosis in myocardial infarction. Report of a workshop on the role of coronary thrombosis in the pathogenesis of acute myocardial infarction. Am J Cardiol 34: 823-833.
- Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92: 657-671.
- Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30: 1282-1292.