Journal of Cardiobiology
Download PDF
Research Article
*Address for Correspondence: med Ernst Wellnhofer, Deutsches Herzzentrum Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, Tel: +493045932498; Fax: +493034901861; E-mail: Wellnhofer@dhzb.de
Citation: Gräfe M, Kriatselis C, Furundzija V, Thanabalasingam U, Gerds-Li JH, et al. Different Role of Left Atrial Size and Function in Patients with Paroxysmal versus Persistent Atrial Fibrillation. Wellnhofer: LA Size & Function in Atrial Fibrillation. J Cardiobiol. 2013;1(2): 10.
Copyright © 2013 Gräfe M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 1, Issue: 2
Submission: 03 October 2013 | Accepted: 10 November 2013 | Published: 15 November 2013
Reviewed & Approved by: Dr. Krasniqi Nazmi, Division of Cardiology, University Hospital of Zurich, Switzerland
Comparison of AFparox to AFpers
Different Role of Left Atrial Size and Function in Patients with Paroxysmal versus Persistent Atrial Fibrillation. Wellnhofer: LA Size & Function in Atrial Fibrillation
Michael Gräfe, Charalampos Kriatselis, Vesna Furundzija, Usan Thanabalasingam, Jin-Hong Gerds-Li, Eckart Fleck and Ernst Wellnhofer*
- Deutsches Herzzentrum Berlin, Augustenburger Platz 1, 13353 Berlin, Germany
*Address for Correspondence: med Ernst Wellnhofer, Deutsches Herzzentrum Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, Tel: +493045932498; Fax: +493034901861; E-mail: Wellnhofer@dhzb.de
Citation: Gräfe M, Kriatselis C, Furundzija V, Thanabalasingam U, Gerds-Li JH, et al. Different Role of Left Atrial Size and Function in Patients with Paroxysmal versus Persistent Atrial Fibrillation. Wellnhofer: LA Size & Function in Atrial Fibrillation. J Cardiobiol. 2013;1(2): 10.
Copyright © 2013 Gräfe M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 1, Issue: 2
Submission: 03 October 2013 | Accepted: 10 November 2013 | Published: 15 November 2013
Reviewed & Approved by: Dr. Krasniqi Nazmi, Division of Cardiology, University Hospital of Zurich, Switzerland
Abstract
Purpose: Pulmonary vein isolation (PVI) is established as interventional treatment in atrial fibrillation (AF). Recent studies suggest that atrial reservoir function (preload) is involved in initiation of paroxysmal AF (AFparox) and conduit function (after-load) may be crucial in persistent AF (AFpers).Methods: We enrolled 336 consecutive patients scheduled for PVI (age 60±10 years). Point-to-point radiofrequency ablation was used in the majority of cases. Success was defined as a minimum of 3 months (median 12 months) follow-up as absence of recurrence of AF in any Holter ECG since PVI.
Results: Patients with recurrent AFparox demonstrated a significantly better outcome (64% success) than patients with AFpers (41% success). AFparox patients were 5 years younger and had smaller atria, a better emptying index, higher systolic pulmonary venous velocity, and only borderline impaired diastolic left ventricular function. LA size per se was not related to success in AFparox. Left ventricular filling times (Tfill) and the ratio of systolic to diastolic tissue Doppler (TDI) velocity (S’/E’) were found to be potential risk predictors. Increased LA-size, reduced LA-emptying and impaired diastolic left ventricular function were found in AFpers patients. S’/E’ and isovolumic relaxation time stratify risk in AFpers patients.
Conclusions: In AFparox long term failure of primary PVI is associated with restrictive reservoir function but not LA enlargement. Whereas in AFpers a combination of severely reduced LA emptying, increased LA size and severely impaired diastolic LV function result in a high rate of recurrent AF. TDI (Tfill, S’/E’) may help to improve risk stratification.
Keywords
Prognosis; Pulmonary vein isolation; Atrial fibrillation; Echocardiography; Diastolic functionIntroduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, particularly among the elderly. AF is responsible for an increased risk of stroke, heart failure and all-cause mortality [1]. Haissaguerre et al. demonstrated that ectopic activity in pulmonary veins (PV) may be responsible for triggering AF, particularly in paroxysmal AF (AFparox) [2]. Thus, ablation of tissue where ectopic activity arises is an interventional treatment strategy. Recent improvements in the technique of catheter ablation provide favorable results compared with pharmacological treatment [3,4]. Therefore, catheter ablation, in particular pulmonary vein isolation (PVI), can be considered as an early option in the management of patients with AF [5]. Success rates of the initial ablation procedure depend on the definition of success; they vary between 60 and 85% for AFparox and are lower for persistent AF (AFpers) [3,6-9].The initiation of AF requires both a trigger and a susceptible substrate. In recurrent AFparox ectopic focal activity is thought to be local and limited to one or a few sites. On the contrary in AFpers there tend to be multiple ectopic sites throughout the atria and advanced damage of atrial myocardium (more susceptible substrate) [10-13]. Large left atrial (LA) size was found to be a predictor of recurrence of AF after electrical or pharmacological cardio-version as well as after ablative treatment [14-17]. The prognostic value of volume seems to be absent in AFparox, however [18].
LA function comprises three phases: reservoir, conduit and active contraction phase [19]. LA- active function increases with slight volume load as long as LA structural dilatation is limited [19,20]. Thus, in early LA dysfunction as expected in AFparox systolic LA dimensions are ambiguous, because an increased systolic volume may reflect increased loading and/or size (history of dilatation).
Recent studies suggest that preserved atrial reservoir function related to preload is a predictor of success [21]. Impairment of atrial reservoir function and poor long-term success of PVI in small left atria was related to enhanced fibrosis [22]. Whereas conduit function related to after-load may become increasingly important for PVI success in large atria with poor emptying.
Thus, we tested the hypothesis that in AFparox atrial reservoir function and in AFpers residual conduit function is crucial for success of PVI by an analysis in the function-size plane in a retrospective study in our PVI data-base. As second end points we wanted to identify echocardiographic parameters potentially predicting recurrence of AF after PVI and elucidate the impact of body mass index (BMI) in this context.
Methods
Study designThe study is a retrospective analysis in the data-base of an observational prospective cohort of consecutive patients scheduled for PVI to treat AF. Inclusion criteria were symptomatic, medically refractory AF eligible for ablative treatment and feasibility of a TTE study. Patients with LA- diameter >60 mm, valve prosthesis, pacemaker or LV-EF < 40% were excluded. The study complies with the declaration of Helsinki. The research protocol was approved by the internal review board and all patients gave written informed consent.
Study protocol
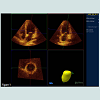
One day prior to the intervention a transthoracic (TTE) and a transoesophageal (TEE) echocardiogram were obtained with an IE33 Philips machine. All patients underwent a comprehensive evaluation with M-mode, B-mode, pulsed Doppler and tissue Doppler imaging. Each measurement was repeated at least seven times in AF and at least thrice if SR was present. Peak early filling velocity (E) and deceleration time (EDT) were determined in pulsed Doppler measurements with a sample volume at the tips of the mitral leaflet. For determination of isovolumic relaxation time (IVRT) the sample volume was adjusted between inflow and outflow jet and time resolution was maximized. LA diameter (LA), area (LAA) and volume (LAV) were evaluated at end systole (maximum) and at end diastole (minimum). LA volumes were calculated from apical LAA by a modified biplane area length method [23]. Emptying index was calculated as 1 - LAVdiast/LAVsys. 4D volumes were additionally acquired by 4D echocardiography (Philips IE33) before PVI since end of 2010. An example is displayed in Figure 1. Pulmonary vein systolic (PVsyst) and diastolic (PVdiast) peak velocities were derived from pulsed Doppler measurements, and a non-dimensional ratio (S/D ratio) was calculated. Systolic (S’) and early diastolic (E’) tissue Doppler (TDI) of left ventricular base/mitral ring velocities were measured in a four chamber (septal and lateral) and two chamber view (anterior, inferior). Median S’, were calculated. TDI data were analyzed offline with QLab™ (PHILIPS B.V Eindhoven The Netherlands). In view of the limitations of LA size and emptying index in predicting PVI success in AFparox we evaluated a high resolution monitoring of the time course left ventricular contraction and filling by measurement of time intervals between R-wave and maximal negative strain and between maximal negative strain and return to 70% of the start value (Tfill). User interaction is minimized (selection of ROI in QLAB™ and visual quality check) to warrant standardized and reproducible assessment of these times. Exported spread sheets are automatically post processed by a simple generic algorithm.
Definition of success
Figure 1: Screenshot of display of 4D-data set QLAB. The contours of the endocardium of the left atrium are seen on the top in a 4 chamber and 2 chamber views. The reconstruction is given on the bottom at the right side. Mimicking left ventricular evaluation implies that the middle roof of the left atrium is marked instead of the left ventricular apex and apparent systole (minimal LA volume) occurs at in end diastole and apparent diastole is maximal end systolic LA-volume.
Follow-up included repeated 24-hour Holter ECG at 1, 3, 6, 9, 12, and 18 months after PVI. Anti-arrhythmic drugs were withdrawn 4 weeks and 3 months after ablative treatment of AFparox and AFpers respectively. Success was defined and assessed independently in patients with a minimum of 3 months follow-up as absence of recurrence of AF in any Holter ECG since PVI.
Ablation procedure
Oral anticoagulation was stopped 3-7 days before the scheduled ablation. Point-to-point radiofrequency ablation was used in the majority of cases in the study sample. Three dimensional anatomy of left atrial pulmonary vein ostia was reconstructed from rotational angiography [24,25]. The end-point of the procedure was the electrical isolation of all pulmonary veins defined as elimination of PV potentials at the Lasso catheter that was positioned as closely as possible to the PV ostium.
Statistics
IBM® SPSS® Statistics version 20, IBM Corporation) was used for statistics. Means ± standard deviations and rates (percentages) and p-values for significant group differences (t-test, Mann Whitney U test) are given. The subset of 4D volumes additionally acquired by 4D echocardiography serves as cross-validation sample. As general validation strategy bootstrapping (100 samples) was performed in larger groups. Correlation and regression analysis and scatter and box plots were employed for pattern analysis regarding group differences. Predictive value was assessed by area under the curve (AUC), crosstable analysis (odds ratio) and binary logistic modeling with Hosmer-Lemeshow test for calibration and bootstrapping for validation. The cut-off estimate for Tfill was determined by a classification and regression tree (CRT) analysis with split-sample validation strategy.
Results
We enrolled 336 consecutive patients (age 60 ± 10 y, 126 female). The impact of age, sex, BMI and history of previous PVI on success in terms of persistent establishment of sinus rhythm in these patients was not significant. During follow-up the majority of patients received additional treatment with drugs acting on rhythm and/or heart rate (88%, 32% >1 drug). Discounting patients treated with β-blockers alone slightly more than 50% of patients received treatment with anti-arrhythmic drugs (< 2% more than one anti-arrhythmic drug). The median follow-up is 1 year. Systolic LV function was normal in the majority of the patients, as reflected by LV fractional shortening. LV diastolic function was compromised. The mean value of E/E’ was elevated, the systolic to diastolic pulmonary venous flow-velocity ratio was decreased and atrial volume was enlarged. An overview of echocardiographic measurements is given in Table 1.PVI in patients with AFparox (N=186, 71 female) demonstrated a significantly (p< 0.001 χ²=14) better long term success (64% success) than in patients with AFpers (N=150, 55 female, 43% success). The odds ratio was 2.36 (confidence interval 1.5-3.7). The patients were about 2 years younger than patients with AFpers. They presented with smaller atria, a comparatively well preserved emptying index, A-wave (active atrial contraction) and borderline or only slightly impaired diastolic left ventricular function as judged by E/E’ and pulmonary vein velocities (see Table 1). The elevated systolic/diastolic pulmonary vein velocity and a preserved A-wave suggest a pathophysiology in these patients that is characterized by increased preload matching, a borderline elevation in after-load due to abnormal left ventricular relaxation. On the contrary, in patients with AFpers a longer history of LV-diastolic dysfunction is suggested by large LA volumes. Loss of active LA-contraction and severely reduced LA-emptying index (after-load mismatch) as well as decreased LA systolic pulmonary vein inflow (preload impairment) highlight global LA-pump failure. Apart from LA-pump failure pathophysiology in these patients is characterized by elevated after-load due to advanced diastolic and early systolic LV dysfunction reflected by a restrictive LV-filling pattern (short early filling and increased E/E’) and a decreased S’/E’ ratio. Particular echocardiography measurements are given in Table 1. The different prognostic potential of atrial size in AFparox as compared to AFpers is illustrated by the ROC-Curves in Figure 2. The main finding is that the prognostic value of LA size by itself is limited to AFpers and principally independent from type of measurement (linear, area, volume).
Figure 2: ROC-curves for different parameters of LA size as predictors of persisting success in paroxysmal AF (top left) and persistent AF (bottom right). Obviously there is no predictive value of LA size in paroxysmal AF irrespective whether measurements are linear area or volume calculations. The predictive value was significant in persistent AF for all size measurements. But, the curve shows that the contribution to prediction is rather poor. More accurate volume measurements (biplane Simpson, 4D-echo or CT/MRI) may improve discrimination in these patients.
PVI success in AFparox
Long-term success of was PVI 64% within this group of patients. Atrial volumes and emptying fraction were slightly smaller in the subgroup with recurrent AF than in the subgroup with persisting sinus rhythm (Table 2 and Figure 3). The difference was not significant, however, and volume was not really helpful in risk stratification. Calculated volumes were larger than 4D TTE volumes, but emptying index was similar. An emptying index above 45% was associated with long-term success and a value below 21% predicted recurrence of AF (see box plot Figure 4). Subgroups of AFparox with respect to success did not show any significant difference with respect to LA after-load as judged by standard parameters of LV diastolic function (E-wave, tissue Doppler E’, E-deceleration time, isovolumic relaxation time) or LA preload (systolic pulmonary vein flow velocity, systolic to diastolic PV flow velocity). Septal S’/E’ was significantly increased in patients with recurrent AF (1.07±0.27 versus 0.97±0.28, p=0.03), however, reflecting contraction/relaxation velocity imbalance (early relaxation failure?). The inter-group difference of S’/E’ was small with large overlap of data. Thus binary logistic regression modeling in combination with maximal systolic (but not end diastolic minimal!) LA-area as covariates was attempted.
Model: P=exp (-0.063*LA area+0.838*S’/E’); odds ratio= 2.1| P<0.05.
Figure 3: This figure displays a scatter-plot with LA emptying index on the y-axis and calculated end-diastolic volumes related to body surface area on the x-axis in patients with paroxysmal AF before PVI. Circles represent persistent success. There is broad overlap between stars and circles, but, it is obvious that in some patients emptying index is rather high and failure is often found if LA is small and/or emptying index is severely impaired.
Figure 4: This box-plot of LA emptying index in patients with paroxysmal AF before PVI analyzes the tails. We compare different quantification methods (shading) and different groups with respect to success (x-axis). Medians, 75% (boxes) and 95% (whiskers) intervals are depicted. Outliers were not removed. The two additional horizontal lines identify a group with high emptying index (>45%) and excellent prognosis (on top) and another group with poor emptying index and high risk of recurrent AF (cut-off: <21%; determined and validated by classification and regression tree analysis (CRT)). For stratification of risk in the intermediate group see Figure 6.
S’/E’ was did not discriminate prognosis on its own, however. Thus, in search of a more straightforward decision model further investigation of LV coupling was performed. In Figure 5 effective diastolic filling time based on off-line analysis of color tissue Doppler data are displayed (see also Table 2). Longer absolute and relative filling times, but not ejection times were associated with persistent success in AFparox. This effect is not explained by higher heart rates (shorter cycle times) or duration of systole. Based on these findings we evaluated a simple decision tree to identify subgroups with worse prognosis based on emptying index (cut-off 21% and 45 %) and Tfill (cut-off 215 ms). As depicted in Figure 6 the groups with residual emptying index <21% or Tfill <215 ms and abnormal emptying index (≤45%) have a worse prognosis. Filtering these subgroups unmasks more than 80% success in AFparox without additional risk. BMI had no modulating impact on long-term success of PVI in AFparox.
PVI success in AFpersIn the AFpers subgroup 57% of PVI procedures necessitated repeat PVI or other strategies due to recurrence of AF. Parameters reflecting LA size (Figure 7 and Table 3) were significantly lower in the subgroup without success. Comparison depends on the method of calculation and/or measurement, however. Particularly 4D LA volumes were generally lower than calculated LA volumes. The emptying index was severely reduced reflecting loss of active LAfunction and distensibility (Figure 8). S’/E’ was significantly decreased in this group due to silent systolic LV-dysfunction (decrease of S’) and in contrast to AFparox in AFpers long-term failure of PVI was associated with even lower S’/E’. Prolonged IVRT in this group is evidence of impaired relaxation. Paradoxically BMI was slightly reduced in AFpers with recurrent AF. In a binary logistic model predicting success BMI performed as significant covariate along with S’/E’, IVRT, LA systolic area in 4CHV.
Model: P=exp (0.139*LA area+0.019* IVRT- 2.507*S’/E’ - 0.12*BMI); odds ratio= 3.7| P<0.05.6Volume cut-off or other LA size cut-off strategies may be used in this group as a simple decision models as shown in Figure 2 on the left side.
Figure 5: Filling times (Tfill TDI septal) stratified with respect to groups (AFparox/AFpers) and success (persisting SR/recurrent AF). Medians, 75% (boxes) and 95% (whiskers) intervals are depicted. Outliers were not removed. The additional horizontal line identifies a group with high risk of recurrent AF (cut-off: <215 ms; determined and validated by classification and regression tree analysis (CRT)). For stratification of risk see Figure 6.
Figure 6: A possible strategy for improving risk stratification in patients with paroxysmal AF before PVI is proposed. Based on the box-plot estimate in Figure 4 in the group with intermediate risk offline analysis of filling times as assessed by tissue Doppler of the left ventricle is performed (for details see methods). Short filling times are associated with increased risk of recurrence of AF. Cut-off values are sample based estimates.
Figure 7: This figure displays a scatter-plot with LA emptying index on the y-axis and calculated end-diastolic volumes related to body surface area on the x-axis in patients with persistent AF before PVI. Recurrent AF is marked by a star. Circles represent persistent success. There is broad overlap between stars and circles, but, it is obvious that in patients with both enlarged volumes and low emptying index the risk of recurrence of AF is particularly high. The horizontal and vertical lines in the plot identify cut-offs (15%/ 60 ml/m²).
Figure 8: This figure displays the impact of volume determination technique (left side calculated volumes, right side 4-D volumes) on emptying index. Data are stratified with respect to groups (AFparox/AFpers) and success (persisting SR/recurrent AF). Despite of methodological limitations of 4D data acquisition in AF this technique may be superior in discriminating risk by emptying index.
Impact of volume determination technique on emptying index
Calculation may be inferior to 4D measurement despite of many limitations of the latter technique in AF. Figure 8 demonstrates that trends in median values and 75% boxes are better correlated with success in 4-data. Further evaluation of this observational finding is necessary, however.
Discussion
We found a progressive decrease in LA function (emptying index and Tfill) related to persistence of AF and success of PVI (see also Figure 8 right side and Figure 5). Only minimally impaired LA function was found in the subgroup of AFparox with PVI success. The subgroup of AFparox with PVI failure is characterized by preserved LA size and moderate impairment of LA-function and/or after-load mismatch. Slight to moderate enlargement of LA is the hallmark of a history of more severe functional impairment in the subgroup of AFpers with successful PVI, whereas in the subgroup of AFpers with PVI failure a burn-out stage comprising poor function and considerable LA enlargement is found. These findings are supported by the small but significant age difference between AFparox and AFpers groups. Increased LA size in AFpers may reflect an advanced stage of LA damage as compared to AFparox or a different pathology between groups(fibrosis with restriction versus dilatation) [22]. An elevated risk of recurrence of AF in AFpers as compared to AFparox is well established [6-8].A conceptual pathophysiological hypothetical framework is proposed to analyze clinical data in this context. The LA may be regarded as a small pump in series between the pulmonary circulation and the left ventricle. For analysis the LA pump cycle is divided into 3 phases and four components [19]. During ventricular systole ventricles become smaller and thus reduce external compressive forces on the left atrium within the pericardial sac. In this reservoir phase filling from the pulmonary veins is facilitated by a suction effect. The left ventricular relaxation generates a gradient between the reservoir and ventricle causing the reservoir to be unloaded and the blood shifted to the ventricle in the passive emptying phase. As the blood flow from the pulmonary veins continues there is an additional conduit volume transport during diastole. At the end of diastole active atrial contraction exerts a booster effect that minimizes LA-volume and provides space for the reservoir filling. Thus a high LA-emptying index and systolic LV-function favor reservoir filling and enhanced reservoir filling in turn stretches LA muscle tissue and improves the emptying index by a Frank-Starling mechanism [23]. As a result reservoir and booster function may act as sufficient compensatory ping-pong mechanism in intermittent increases of after-load predominantly due to early LVdiastolic dysfunction. Bouts of AF in AFparox patients may indicate exhaustion of this adaptive coupling mechanism. Our data support the hypothesis that persistent success of single PVI in patients with AFparox implies preservation of this mechanism and coping with after-load. Shortening of effective filling times of the left ventricle (Tfill) and relative decrease in E’ reflected by increased S’/E’ and/or a decreased emptying index reflect a decrease in LV relaxation and/or LA after-load mismatch. Impairment of function was shown to precede structural changes (enlargement) of the LA in a recent study by velocity imaging vector echocardiography [26]. The combination of small volumes with a decreased emptying index in patients with AF parox strongly suggests restrictive atrial disease as potential cause of LA after-load mismatch [22]. Atrial fibrosis was proposed as risk factor for recurrence of atrial fibrosis based on a magnetic resonance imaging study recently [27]. Speckle strain imaging of the LA to may help to quantify atrial fibrosis in echocardiography and is currently evaluated [21]. Functional impairment as reflected by emptying index and effective filling times provides a straightforward decision tree for risk stratification as depicted in Figure 6 for this group.
LA size in patients with AF perm is morphological evidence of a history of LA after-load mismatch and diastolic LV dysfunction. LA volume is a fair parameter for risk stratification in AFpers [14-17]. A low emptying index and loss of A-wave indicate that reservoir and booster function are compromised. The large LA blunts the reduction of external compressive forces within the pericardial sac during systole. Thus an increase of diastolic pulmonary vein flow and filling pressure in the conduit phase is the only remaining mechanism to adjust coupling with the LV. This mechanism operates at risk of pulmonary congestion, as any increase in LV filling pressure needs to be counter-balanced by a respective increase in diastolic pulmonary wedge pressure. Thus emptying during the conduit phase largely depends on after-load primarily determined by left ventricular (LV) diastolic function, which was found to be an important outcome predictor after intraoperative ablation for atrial fibrillation [28]. In our study population conventional parameters of diastolic function apart from IVRT such as mitral annulus tissue and in-flow Doppler velocities did not show any predictive value related to success of PVI. This finding may be due to low sensitivity and inherent limitations by well known methodological confounders, e.g. bad alignment of wall motion with the insonation angle. Septal S’/E’ a non-dimensional parameter that does not depend on insonation angle was found to be severely decreased in patients suffering from AFpers with recurrent AF. And BMI is an significant probably not causal covariate in the risk model. Further evaluation of logistic models comprising LA systolic area (or volume), IVRT, S’/E’ and BMI need further evaluation. Whether Tfill is useful in this context has to be evaluated in a larger sample size.
In terms of risk prediction additionally right atrial AF drivers [28-30] might be assessed by evaluation of temporal and spatial synchronicity of cyclic bi-atrial strain.
BMI was not significantly different between groups and subgroups. Its role in the logistic prediction model in AFpers is probably related to parameter adjustment for body size. Whereas correlation analysis suggested independence of LA area, IVRT and S’/E’, BMI was related to atrial size and diastolic filling. Moreover, there was a borderline significant difference in height between the group with SR and recurrent AF in AFpers.
Limitations
Anatomy, function and coupling of the LA, as assessed by echocardiography, are only one factor complex in predicting outcome of ablation. Other factors as scar burden (magnetic resonance imaging), history, co-morbidities procedural factors eluding quantification play an important role [31]. Current limitations of PVI techniques are held to be a major cause of treatment failure [31,32]. Furthermore concomitant medical therapy was not controlled.This study is observational, mono-centric and was retrospectively analyzed. The data were prospectively collected, however, and repeat sampling and bootstrapping were performed to exclude accidental findings. Though the results of this observational study do not prove causal inference they support hypotheses that need further testing.
A major limitation is the choice of the endpoint reflected by high event rates due to late recurrence of AF. A Kaplan Meier or Cox regression approach to adjust for time of follow-up was not usedin this observational study. The resulting loss in sensitivity of the endpoint is not detrimental, however, for hypothesis generation. A Kaplan Meier statistic may be used to evaluate the prediction models including TDI parameters pre-specified in this paper in a larger prospective sample.
4D echocardiography provided superior LA volume data in our sample than calculation of LA volumes from 2D data in terms of risk stratification for PVI (see Figure 8). Though the 4D subsample is rather small 4D data reproduce and confirm 2D trends and results. Further investigation is needed to evaluate whether 4D is really superior to 2D in this context (AF) as suggested by our data. Stitching artifacts might be a problem for 4D echocardiography in patients with AF. New real time 4D systems are on the market and particularly for patients with AF real time high frequency large volume 4D echocardiography promises an advance in LA volume assessment.
Conclusion
In AFparox atrial reservoir function and in AFpers residual conduit function seems crucial for success of PVI. Left atrial volume or area measurements are not suitable for risk stratification in patients with paroxysmal atrial fibrillation. A further improvement of risk stratification in this group may be feasible by assessing restrictive impairment in left atrial strain. Prediction models including nondimensional or temporal TDI parameters that reflect left ventricular filling need prospective validation. BMI does not seem to be a major independent risk factor for recurrence of atrial fibrillation. Further studies may define the role of additional parameters reflecting right atrial function and atrial asynchrony that were lacking in our echocardiographic protocol.Acknowledgements
We thank Mrs. A. Gale, ELS, for editorial assistance.References
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE (1995) The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98: 476-484.
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, et al. (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339: 659-666.
- Bonanno C, Paccanaro M, La Vecchia L, Ometto R, Fontanelli A (2010) Efficacy and safety of catheter ablation versus antiarrhythmic drugs for atrial fibrillation: a meta-analysis of randomized trials. J Cardiovasc Med (Hagerstown) 11: 408-418.
- Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, et al. (2006) A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 48: 2340-2347
- Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, et al. (2008) Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation. Health Technol Assess 12: iii-iv, xi-xiii, 1-198.
- Gaita F, Caponi D, Scaglione M, Montefusco A, Corleto A, et al. (2008) Long-term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circ Arrhythm Electrophysiol 1: 269-275.
- Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, et al. (2002) Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 105: 1077-1081.
- Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, et al. (2010) Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 3: 237-242.
- Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, et al. (2011) Catheter ablation for atrial fibrillation are results maintained at 5 years of follow-up? J Am Coll Cardiol 57: 160-166.
- Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, et al. (2007) The efficacy of inducibility and circumferential ablation with pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 18: 607-611.
- Di Biase L, Elayi CS, Fahmy TS, Martin DO, Ching CK, et al. (2009) Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol 2: 113-119.
- Knecht S, Hocini M, Wright M, Lellouche N, O'Neill MD, et al. (2008) Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J 29: 2359-2366.
- Willems S, Drewitz I, Steven D, Hoffmann BA, Meinertz T, et al. (2010) [Interventional therapy of atrial fibrillation: possibilities and limitations]. Dtsch Med Wochenschr 135: S48-S54.
- Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, et al. (2009) Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 11: 1289-1294.
- Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, et al. (2009) Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol 20: 1005-1010.
- Jahnke C, Fischer J, Mirelis JG, Kriatselis C, Gerds-Li JH, et al. (2011) Cardiovascular magnetic resonance imaging for accurate sizing of the left atrium: predictability of pulmonary vein isolation success in patients with atrial fibrillation. J Magn Reson Imaging 33: 455-463.
- Lo LW, Lin YJ, Tsao HM, Chang SL, Udyavar AR, et al. (2009) The impact of left atrial size on long-term outcome of catheter ablation of chronic atrial fibrillation. J Cardiovasc Electrophysiol 20: 1211-1216.
- von Bary C, Dornia C, Eissnert C, Nedios S, Roser M, et al. (2012) Predictive value of left atrial volume measured by non-invasive cardiac imaging in the treatment of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 34: 181-188.
- Pagel PS, Kehl F, Gare M, Hettrick DA, Kersten JR, et al. (2003) Mechanical function of the left atrium: new insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiology 98: 975-994.
- Payne RM, Stone HL, Engelken EJ (1971) Atrial function during volume loading. J Appl Physiol 31: 326-331.
- Mirza M, Caracciolo G, Khan U, Mori N, Saha SK, et al. (2011) Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study. J Interv Card Electrophysiol 31: 197-206.
- den Uijl DW, Delgado V, Bertini M, Tops LF, Trines SA, et al. (2011) Impact of left atrial fibrosis and left atrial size on the outcome of catheter ablation for atrial fibrillation. Heart 97: 1847-1851.
- Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, et al. (2008) Three methods for evaluation of left atrial volume. Eur J Echocardiogr 9: 351-355.
- Kriatselis C, Tang M, Nedios S, Roser M, Gerds-Li H, et al. (2009) Intraprocedural reconstruction of the left atrium and pulmonary veins as a single navigation tool for ablation of atrial fibrillation: a feasibility, efficacy, and safety study. Heart Rhythm 6: 733-741.
- Kriatselis C, Tang M, Roser M, Fleck E, Gerds-Li H (2009) A new approach for contrast-enhanced X-ray imaging of the left atrium and pulmonary veins for atrial fibrillation ablation: rotational angiography during adenosine-induced asystole. Europace 11: 35-41.
- Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, et al. (2012) Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging 13: 227-234.
- Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, et al. (2011) Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 22: 16-22.
- Houltz B, Johansson B, Berglin E, Karlsson T, Edvardsson N, et al. (2010) Left ventricular diastolic function and right atrial size are important rhythm outcome predictors after intraoperative ablation for atrial fibrillation. Echocardiography 27: 961-968.
- Hocini M, Nault I, Wright M, Veenhuyzen G, Narayan SM, et al. (2010) Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol 55: 1007-1016.
- Rostock T, Steven D, Hoffmann B, Servatius H, Drewitz I, et al. (2008) Chronic atrial fibrillation is a biatrial arrhythmia: data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circ Arrhythm Electrophysiol 1: 344-353.
- Kowal RC (2012) PVI’s inconvenient truths: lights out for dormant reconnection? J Cardiovasc Electrophysiol 23: 261-263.
- Shah D (2011) A critical appraisal of cardiac ablation technology for catheter-based treatment of atrial fibrillation. Expert Rev Med Devices 8: 49-55.