Journal of Bioelectronics and Nanotechnology
Download PDF
Research Article
*Address for Correspondence: Hani A. Alhadrami, Faculty of Applied Medical Sciences, Department of Medical Laboratory Technology and Special Infectious Agents Unit, King Fahd Medical Research Centre, King Abdulaziz University, P. O. Box 80402, Jeddah, 21589, Kingdom of Saudi Arabia, Tel: +966505545275; E-mail: hanialhadrami@kau.edu.sa
Citation: Alhadrami HA, Al-Hazmi F. Antibacterial Activities of Titanium Oxide Nanoparticles. J Bioelectron Nanotechnol 2017;2(1): 5.
Copyright © 2017 Alhadrami HA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Bioelectronics and Nanotechnology | Volume: 2, Issue: 1
Submission: 26 February, 2017 | Accepted: 22 March, 2017 | Published: 28 March, 2017

Where D is the average dimensions of crystallites, β is the broadening of the diffraction line measured at half maximum intensity, λ is the wave length of the x-ray radiation and θ is the Bragg angle. Based on peak broadening (Figure 3), the calculated crystallite size of as prepared TiO2 is found to be of the order of 17 nm. Fourier Transformation Spectroscopy (FTIR) analysisFTIR spectroscopy was used in the range of 400-4000 cm-1 to assess the chemical composition and the quality of the synthesized TiO2 nanoparticles. FTIR spectrum results of the synthesized TiO2 nanorods were presented in (Figure 4). The results showed the presence of sharp and strong two bands at 526 and 585 cm-1. This was due to the formation of the stretching vibration of metal-oxygen (Ti-O) bonds. This reveals that the synthesized products were pure TiO2. Further, the presence of a band located at 2340 cm-1 was due to the characteristic absorption of TiO2. In summary, the FTIR spectra confirmed that the synthesized TiO2 nanoparticles composed of one phase, which agrees with X-ray analysis. Optical properties of TiO2 nanorodsThe energetic bands structure and the energy band gap of the prepared TiO2 nanorods have been determined using optical absorption spectra. The absorption coefficient (α) is related to the band gap (Eg) by the following equation (Assuming the band gap to be parabolic in nature):
Where (h) is the plank constant, (υ) is the photo frequency, (B) is a constant and (n) is the transition type. The absorption coefficient (α) can be extracted from: Where (I) is the intensity of transmitted light, (I0) is the intensity of the incident light, and (t) is the thickness of the sample.The optical band gap (Eg) of the synthesized TiO2 nanorods was calculated from plotting (αhυ) versus (hυ) as illustrated in (Figures 3). The linearity of plotting (αhυ)2 on the y-axis versus (hυ) on the x-axis was interpolated to cut x-axis corresponding to y = 0. The intercept on the X-axis is a measure of the Eg of the tested samples. As seen in (Figures 5), the optical band energy gap is about 2.3 eV. TGA analysisThe Thermogravimetric (TGA) analysis of the synthesized TiO2 was presented in (Figures 6). The results confirmed two distinctive weight loss steps (Figures 6). The first weight loss happened at 100-250 °C, which paralleled to the evaporation of crystallized water. The second weight loss took place at around 260-700 °C, leading to the conversion of anhydrous titanium to titanium oxide. Photoluminescence spectroscopyPreviously reported results showed that the indirect gap nanostructure semiconductors can have luminescence because of quantum confinement. Accordingly, many researchers were directed to explore the optical and optoelectronic properties of indirect gapnanostructures. Figures 7 showed photoluminescence measured at room temperature excited by energy chosen at the wavelength corresponding to the position of the minimum appeared in the first derivative of absorption spectrum. The luminescence spectrum of the nanotube consists of high energy emission in blue region of visible light with high intensity at 404 nm along with low intensity shoulder at 464 nm. The emission at 404 nm was at energy slightly less than the value of the determined band edge, which could be attributed to free excitons near to the band edge. The low intensity shoulder at 464 nm may be attributed to Ti-OH complex existing at the surface of nanotube, which induces surface states in the band gap of nanotubes. Antibacterial activity of TiO2 nanorodsThe antibacterial activity of TiO2 nanorods tested against E. coli ATCC® 8739™ is presented in (Figures 8). The viable bacteria were monitored by counting the number of Colony Forming Units (CFUs). The presented results are an average of triplicate measurements and a negative control (TiO2 nanorods incubated with LB broth). Statistical analysis was performed using Minitab 15 for Windows to compare the number of CFUs/ml during different exposure time to TiO2 nanorods (0, 30 min and 24 h). The results revealed a significant reduction (p<0.05) in the number of CFUs/ml after only 30 min of exposure to TiO2 nanorods (Figures 8). There was a significant difference between the number of CFUs/ml after 0 min exposure to TiO2 nanorods and the number of CFUs/ml after 30 min exposure. A growth inhibition of three orders of magnitude is observed after only 30 min of exposing E. coli to the TiO2 nanorods. Further, a significant decrease (p<0.05) is observed in the number of CFU s/ml for E. coli after 24 h exposure to the TiO2 nanoparticles (Figures 8). Taken together these results, there was superior antibacterial activity of the synthesized TiO2 nanorods against the pathogenic bacteria E. coli, which demonstrates potential applications of TiO2 nanorods in medical and biomedical fields.
Where (I) is the intensity of transmitted light, (I0) is the intensity of the incident light, and (t) is the thickness of the sample.The optical band gap (Eg) of the synthesized TiO2 nanorods was calculated from plotting (αhυ) versus (hυ) as illustrated in (Figures 3). The linearity of plotting (αhυ)2 on the y-axis versus (hυ) on the x-axis was interpolated to cut x-axis corresponding to y = 0. The intercept on the X-axis is a measure of the Eg of the tested samples. As seen in (Figures 5), the optical band energy gap is about 2.3 eV. TGA analysisThe Thermogravimetric (TGA) analysis of the synthesized TiO2 was presented in (Figures 6). The results confirmed two distinctive weight loss steps (Figures 6). The first weight loss happened at 100-250 °C, which paralleled to the evaporation of crystallized water. The second weight loss took place at around 260-700 °C, leading to the conversion of anhydrous titanium to titanium oxide. Photoluminescence spectroscopyPreviously reported results showed that the indirect gap nanostructure semiconductors can have luminescence because of quantum confinement. Accordingly, many researchers were directed to explore the optical and optoelectronic properties of indirect gapnanostructures. Figures 7 showed photoluminescence measured at room temperature excited by energy chosen at the wavelength corresponding to the position of the minimum appeared in the first derivative of absorption spectrum. The luminescence spectrum of the nanotube consists of high energy emission in blue region of visible light with high intensity at 404 nm along with low intensity shoulder at 464 nm. The emission at 404 nm was at energy slightly less than the value of the determined band edge, which could be attributed to free excitons near to the band edge. The low intensity shoulder at 464 nm may be attributed to Ti-OH complex existing at the surface of nanotube, which induces surface states in the band gap of nanotubes. Antibacterial activity of TiO2 nanorodsThe antibacterial activity of TiO2 nanorods tested against E. coli ATCC® 8739™ is presented in (Figures 8). The viable bacteria were monitored by counting the number of Colony Forming Units (CFUs). The presented results are an average of triplicate measurements and a negative control (TiO2 nanorods incubated with LB broth). Statistical analysis was performed using Minitab 15 for Windows to compare the number of CFUs/ml during different exposure time to TiO2 nanorods (0, 30 min and 24 h). The results revealed a significant reduction (p<0.05) in the number of CFUs/ml after only 30 min of exposure to TiO2 nanorods (Figures 8). There was a significant difference between the number of CFUs/ml after 0 min exposure to TiO2 nanorods and the number of CFUs/ml after 30 min exposure. A growth inhibition of three orders of magnitude is observed after only 30 min of exposing E. coli to the TiO2 nanorods. Further, a significant decrease (p<0.05) is observed in the number of CFU s/ml for E. coli after 24 h exposure to the TiO2 nanoparticles (Figures 8). Taken together these results, there was superior antibacterial activity of the synthesized TiO2 nanorods against the pathogenic bacteria E. coli, which demonstrates potential applications of TiO2 nanorods in medical and biomedical fields.
Antibacterial Activities of Titanium Oxide Nanoparticles
Hani A. Alhadrami1,2* and Faten Al-Hazmi3
- 1Faculty of Applied Medical Sciences, Department of Medical Laboratory Technology, King Abdulaziz University, Kingdom of Saudi Arabia
- 2Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Kingdom of Saudi Arabia
- 3Department of Physics, King Abdulaziz University, Kingdom of Saudi Arabia
*Address for Correspondence: Hani A. Alhadrami, Faculty of Applied Medical Sciences, Department of Medical Laboratory Technology and Special Infectious Agents Unit, King Fahd Medical Research Centre, King Abdulaziz University, P. O. Box 80402, Jeddah, 21589, Kingdom of Saudi Arabia, Tel: +966505545275; E-mail: hanialhadrami@kau.edu.sa
Citation: Alhadrami HA, Al-Hazmi F. Antibacterial Activities of Titanium Oxide Nanoparticles. J Bioelectron Nanotechnol 2017;2(1): 5.
Copyright © 2017 Alhadrami HA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Bioelectronics and Nanotechnology | Volume: 2, Issue: 1
Submission: 26 February, 2017 | Accepted: 22 March, 2017 | Published: 28 March, 2017
Abstract
Titanium dioxide nanostructures are promising material for optical and antibacterial applications. In this study, TiO2 nanoparticles have been synthesized using facile microwave-assisted hydrothermal process. The optical properties and the structure of the synthesized TiO2 nanoparticles were characterized using several techniques such as Transmission Electron Microscopy (TEM), UV measurements, Fourier Transform Infrared (FTIR), Energy and Dispersive X-ray Spectroscopy (EDXS), X-ray diffraction, Scanning Electron Microscopy (SEM), and Thermogravimetric (TGA). The antibacterial activity of TiO2 nanoparticles were assessed against the pathogenic strain E. coli ATCC® 8739™. The reported results indicated superior antibacterial activity of TiO2 nanoparticles tested against the pathogenic bacteria, which reveals potential applications of TiO2 nanoparticles in biomedical and medical fields.Keywords
TiO2 nanorods; Optical properties; Microwave assisted; Antibacterial activityIntroduction
Photocatalysis is essentially an advanced oxidation process, which has proved to be an effective approach to degrade a wide range of organic, inorganic compounds and toxic metal ions, and to kill microorganisms and viruses. In addition, photocatalysis is an entirely green technology, which does not require or consume any chemical additives (some photocatalytic applications involve other oxidants such as hydrogen peroxide H2O2 to enhance the efficiency). Thus, this emerging technology has demonstrated great promise for environmental applications such as water treatment and air purification. In the recent years, photocatalysis has been employed for water treatment to remove organic and inorganic molecules from water and gas. Fujishima et al. reported that photocatalysis is able to divide water into hydrogen and oxygen [1]. ZnO, Fe2O3, MgO, WO3, Bi2WO6, Bi2O32 are the most semiconductors that can be used for photocatalysis and safe materials for human beings, animals and plants [2]. These attractive features enable these materials to be applied in different applications such as chemical sensors, medicine and water treatment. Recently Hunge et al. have prepared tungsten trioxide (WO3) thin films using spray pyrolysis technique and used it for the degradation of methyl orange. They reported that the degradation rate of methyl orange was over 98% using WO3 photoelectrodes which confirms that it can be employed for applications of water purification [3].Titanium dioxide (TiO2) nanoparticles have been commonly employed as photocatalysts because TiO2 is biologically and chemically stable, corrosion-resistive and inexpensive [4]. TiO2 is abundance in nature, non-toxic with great optical transparency and refractive index and wide band gap energy [5]. One dimensional TiO2 nanostructures with different morphologies prepared by different techniques, such as nanorods, nanowires and nanotubes structures [6-9]. In recent years, TiO2 nanostructures have been synthesized using various methods such as sol-gel, hydrothermal, solvothermal, pulsed laser deposition, Chemical Vapor Deposition (CVD), electrodeposition, Microwave method, spray pyrolysis and sputtering [5,10-14]. Among these methods, spray pyrolysis technique has been widely used for several environmental applications [5]. TiO2-activated carbon nanocomposite was synthesized using a sol-gel method to study photocatalytic degradation of reactive red 198, and it was reported that nanocomposite enhanced the photocatalytic activity of TiO2 nanoparticles [4]. Hunge et al. have prepared stratified WO3/ TiO2 thin films and reported that the film can be used for treatment of polluted water [4]. Microwave heating method is attractive due to its capability of generating fast bulk heating which can result in high reaction rates during short reaction time, and massive product yield in comparison with conventional heating technologies [15-19]. Hence, microwave technology is a promising technology for the development of the green chemistry and it is an essential technology for synthesizing of nanostructures with versatile morphology and uniform particle size [20-27].Wound infection is considered as a global problem of human health and contributes significantly to the escalation in the cost of health care [28]. Wound infection is classified as acute or chronic based on the natural repair process [29]. Antibiotics are the most preferred therapeutic agent to treat and prevent wound infection [28]. Although antibiotics are effective in treating the bacterial infection, improper use of antibiotics creates multidrug resistance microorganisms [30]. Increase prevalence of antibiotic resistance with less development in the antibiotic field resulting in the shortage of novel classes of the antibiotic agent that used to eliminate multidrug-resistant bacteria [31]. In recent years, researchers start to look for the alternative safe treatment which can be employed instead of conventional antibiotic for wound infection. Currently, there are some noble metallic nanoparticles have an effective antibacterial activity such as zinc, copper, titanium and silver [30,31]. One of the most attractive nanoparticles that can be used for the treatment of wound infection is titanium dioxide (TiO2) nanoparticles [27]. TiO2 nanoparticles have been recently employed in medical applications due to their attractive features such as versatile surface chemistry, highly controllable absorption, improve tissue penetration and reduce photochemical damage [2]. Introducing novel TiO2 nanoparticles as antibacterial agents can control the mortality and morbidity rate of the infectious diseases.In this study, we synthesized TiO2 nanorods using a facile microwave-assisted hydrothermal process taking into consideration several parameters such as, time, temperature, and concentration of basic solution on the growth of nanocrystals. The structural of the produced nanoparticles have been characterized using XRD, SEM, EDS, FTIR, UV and TEM. Optical, thermal and antibacterial properties of TiO2 nanorods were addressed in detail.Materials and Methods
Synthesis of TiO2 nanorodsTiO2 nanorods have been synthesized using wet chemical process with microwave-assisted. Two chemical processes were used to control the synthesize of TiO2 nanorods. The first was a wet chemical route assessment of microwave irradiation and hydrothermal technique. The second was based on a simple reaction with water to synthesis the proposed TiO2 nanorods. The synthesis process involved using 25 ml of Titanium (IV) tetrachloride tetrachloride (TiCl4, 99.9%, purchased from Aldrich), and 50 ml of water. Microwave hydrothermal technique was used as follows: Titanium tetrachloride was mixed with distilled water. The mixture was transferred into Teflon-lined autoclave. The autoclave was sealed and maintained to 220 °C for 90 min in microwave furnace with a power 700 w, then left to cool down at room temperature. Precipitates were collected by centrifugation at 3000 rpm for 5 min, and then a nomination with distilled water was applied to reduce the agglomeration. The mixture was dried at 80 °C for 3 h and the white powder of TiO2 nanorods was finally generated.Antibacterial activity of TiO2 nanorodsE. coli ATCC® 8739™ was grown overnight in 250 ml Erlenmeyer flask containing 100 ml Luria Bertani (LB) broth, and incubated in 150 rpm orbital shaker incubator at 37 °C. The overnight culture was harvested by centrifugation at 6000 rpm for 5 minutes. To remove any bound organic and inorganic components, the cells were washed twice with normal saline (0.85% NaCl solution at pH 6.5). The washed cells were re-suspended in 4 ml normal saline. TiO2 nanorods were washed with 70% ethanol and left to dry for 5 min. The bacterial suspension was incubated with 100 mg TiO2 nanorods and incubated overnight at 37 °C in 150 rpm orbital shaker incubator. An aliquot (100 μl) was taken at different time intervals (0, 30 min and 24 h), diluted and plated on LB agar plates to determine the number of colony forming units (CFUs/ml). Counts of CFUs were performed by applying the drop-plate method. Serial dilutions (1:10) were achieved by diluting the bacterial cells (100 μl) in micro-centrifuge tubes containing 25% normal saline (900 μl). Three 10 μl aliquots of the appropriate dilution were spotted onto LB agar plate and incubated overnight at 37 °C. The experiment was performed in triplicate, and negative control (TiO2 nanorods incubated with LB broth) was used.Results and Discussion
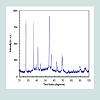
SEM and TEM results of TiO2 nanorodsSize and morphology of the synthesized TiO2 nanorods were examined by SEM. High and low SEM magnification images are illustrated in (Figures 1a and 1b) respectively. It is clearly noticeable in (Figure 1c) that the obtained products have been successfully synthesized in large quantity and retaining nanorod-like morphologies. EDS spectra of the synthesized TiO2 nanorods was represented in (Figure 1d), which indicated the existence of oxygen and Mg. TME was used to demonstrate the clear morphologies of the synthesized TiO2 nanorods (Figure 2). The results confirmed that the TiO2 had uniform nanorods and are grown in large scale. X-ray studies of TiO2 nanorodsThe typical X- ray diffraction pattern of TiO2 nanorods synthesized by microwave technique is presented in (Figure 3). The X- ray diffraction pattern confirmed that TiO2 had a polycrystalline structure. The significant peaks for the pristine TiO2 were observed at 2=27.62°, 36.21°, 38.37°, 41.37°, 44.60°, 54.45°, 56.85°, 63.05°, 65.06° and 69.17°, which could be indexed as (110), (101), (200), (111), (210), (211), (220), (002), (310) and (301), respectively based on JCPDS card No. 01-089-4920. The peaks are fully assigned to TiO2 and their broad width suggests the presence of small-sized particles. The crystal structure of the TiO2 is rutile tetragonal structure with lattice parameter of a=0.4512 nm and c=0.2943 nm. The average size of crystallites was determined based on X-ray peak broadening the (101) reflection using the Scherrer formula: The typical X- ray diffraction pattern of TiO2 nanorods synthesized by microwave technique is presented in (Figure 3). The X- ray diffraction pattern confirmed that TiO2 had a polycrystalline structure. The significant peaks for the pristine TiO2 were observed at 2=27.62°, 36.21°, 38.37°, 41.37°, 44.60°, 54.45°, 56.85°, 63.05°, 65.06° and 69.17°, which could be indexed as (110), (101), (200), (111), (210), (211), (220), (002), (310) and (301), respectively based on JCPDS card No. 01-089-4920. The peaks are fully assigned to TiO2and their broad width suggests the presence of small-sized particles. The crystal structure of the TiO2 is rutile tetragonal structure with lattice parameter of a=0.4512 nm and c=0.2943 nm. The average size of crystallites was determined based on X-ray peak broadening the (101) reflection using the Scherrer formula:Where D is the average dimensions of crystallites, β is the broadening of the diffraction line measured at half maximum intensity, λ is the wave length of the x-ray radiation and θ is the Bragg angle. Based on peak broadening (Figure 3), the calculated crystallite size of as prepared TiO2 is found to be of the order of 17 nm. Fourier Transformation Spectroscopy (FTIR) analysisFTIR spectroscopy was used in the range of 400-4000 cm-1 to assess the chemical composition and the quality of the synthesized TiO2 nanoparticles. FTIR spectrum results of the synthesized TiO2 nanorods were presented in (Figure 4). The results showed the presence of sharp and strong two bands at 526 and 585 cm-1. This was due to the formation of the stretching vibration of metal-oxygen (Ti-O) bonds. This reveals that the synthesized products were pure TiO2. Further, the presence of a band located at 2340 cm-1 was due to the characteristic absorption of TiO2. In summary, the FTIR spectra confirmed that the synthesized TiO2 nanoparticles composed of one phase, which agrees with X-ray analysis. Optical properties of TiO2 nanorodsThe energetic bands structure and the energy band gap of the prepared TiO2 nanorods have been determined using optical absorption spectra. The absorption coefficient (α) is related to the band gap (Eg) by the following equation (Assuming the band gap to be parabolic in nature):
Where (h) is the plank constant, (υ) is the photo frequency, (B) is a constant and (n) is the transition type. The absorption coefficient (α) can be extracted from:
Conclusion
In this work, performance photocatalysts TiO2 nanorods were successfully synthesized using microwave-assisted hydrothermal process. The SEM, EDX, TEM and XRD analysis indicated that photocatalysts TiO2 nanorods are crystalline. Moreover, photocatalysts TiO2 nanorods using water as a solvent via a facile microwave-assisted hydrothermal process were prepared in this work for the first time. The TiO2 nanorods have an effective antibacterial ability against Escherichia coli (Gram negative), which makes TiO2 nanorods very useful as antibacterial agent and in many biomedical applications. Finally, the one outstanding feature of this study is that we need more experimental work under different growth conditions to which we could demonstrate that photocatalysts metal oxide nanostructures are promising for important applications in water purification.Acknowledgements
The project was funded by Research Endowment Fund (WAQF), King Abdulaziz University, under grant 1435-1436. The authors, therefore, acknowledge with thanks WAQF financial support.References
- Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238: 37-38.
- Wang M, Ioccozi J, Sun L, Lin C, Zhiqun Lin (2014) Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ Sci 7: 2182-2202.
- Hunge YM, Mahadik MA, Kumbhar SS, Mohite VS, Rajpure KY et al. (2016) Visible light catalysis of methyl orange using nanostructured WO3 thin films. Ceram Int 42: 789-798.
- Hunge YM, Mahadik MA, Moholkar AV, Bhosale CH (2017) Photoelectrocatalytic degradation of oxalic acid using WO3 and stratified WO3/TiO2 photocatalysts under sunlight illumination. Ultrason Sonochem 35(Pt A): 233-242.
- Mohite VS, Mahadik MA, Kumbhar SS, Hunge YM, Kim HJ, et al. (2015) Photoelectrocatalytic degradation of benzoic acid using Au doped TiO2 thin films. J Photochem Photobiol B 142: 204-211.
- Varghese OK, Gong D, Paulose M, Ong KG, Dickey EC, et al. (2003) Extreme changes in the electrical resistance of titania nanotubes with hydrogen exposure. Adv Mater 15: 624-627.
- Wen B, Liu C, Liu Y (2005) Depositional characteristics of metal coating on single-crystal TiO2 nanowires. J Phys Chem B 109: 12372-12375.
- Wu JM, Qi B (2007) Low-temperature growth of a nitrogen-doped titania nanoflower film and its ability to assist photodegradation of rhodamine B in water. J Phys Chem C 111: 666-673.
- Yi GR, Moon JH, Yang SM (2001) Ordered macroporous particles by colloidal templating. Chem Mater 13: 2613-2618.
- Andersson M, Oesterlund L, Ljungstroem S, Palmqvist A (2002) Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J Phys Chem B 106: 10674-10679.
- Wahi RK, Liu Y, Falkner JC, Colvin VL (2006) Solvothermal synthesis and characterization of anatase TiO2 nanocrystals with ultrahigh surface area. J Colloid Interface Sci 302: 530-536.
- Bazargan MH, Byranvand MM, Kharat AN (2012) Preparation and characterization of low temperature sintering nanocrystalline TiO2 prepared via the sol-gel method using titanium (IV) butoxide applicable to flexible dye sensitized solar cells. Int J Mat Res 103: 347-351.
- Shinde PS, Bhosale CH (2008) Properties of chemical vapour deposited nanocrystalline TiO2 thin films and their use in dye-sensitized solar cells. J Anal Appl Pyrolysis 82: 83-88.
- Arami H, Mazloumi M, Khalifehzadeh R, Sadrnezhaad SK (2007) Sonochemical preparation of TiO2 nanoparticles. Mater Lett 61: 4559-4561.
- Fang X, Zhang L (2006) One-dimensional (1D) ZnS nanomaterials and nanostructures. J Mater Sci Technol 22: 721-736.
- Cui Z, Meng GW, Huang WD, Wang GZ, Zhang LD (2000) Preparation and characterization of MgO nanorods. Mater Res Bull 35: 1653-1659.
- Yin Y, Zhang G, Xia Y (2002) Synthesis and characterization of MgO nanowires through a vapor-phase precursor method. Adv Funct Mater 12: 293-298.
- Zhang J, Zhang L, Peng X, Wang X (2001) Fabrication of MgO nanobelts using a halide source and their structural characterization. Appl Phys A 73: 773-775.
- Shah MA, Haq Q (2009) J Alloys Compd 442: 548.
- Al-Hazmi F, Umar A, Dar GN, Al-Ghamdi AA, Al-Sayari SA, et al. (2012) Microwave assisted rapid growth of Mg(OH)2 nanosheet networks for ethanol chemical sensor application. J Alloys Compd 519: 4-8.
- Umar A, Al-Hazmi F, Dar GN, Zaidi SA, Al-Tuwirqi RM, et al. (2012) Ultra-sensitive ethanol sensor based on rapidly synthesized Mg(OH)2 hexagonal nanodisks. Sens Actuators B Chem 166-167: 97-102.
- Beall GW, Duraia EM, El-Tantawy F, Al-Hazmi F, Al-Ghamdi AA (2013) Rapid fabrication of nanostructured magnesium hydroxide and hydromagnesite via microwave-assisted technique. Power Technol 234: 26-31.
- Al-Ghamdi AA, Al-Hazmi F, Alnowaiser F, Al-Tuwirqi RM, Al-Ghamdi AA (2012) A new facile synthesis of ultra fine magnesium oxide nanowires and optical properties. J Electroceram 29: 198-203.
- Aal NA, Al-Hazmi F, Al-Ghamdi AA, Al-Ghamdi A, El-Tantawy F, et al. (2015) Novel rapid synthesis of zinc oxide nanotubes via hydrothermal technique and antibacterial properties. Spectrochim Acta A Mol Biomol Spectrosc 135: 871-877.
- Al-Ghamdi AA, Al-Hazmi F, Al-Hartomy OA, El-Tantawy F, Yakuphanoglu F (2012) A novel synthesis and optical properties of cuprous oxide nano octahedrons via microwave hydrothermal route. J Solgel Sci Technol 63: 187-193.
- Al-Hazmi F, Alnowaiser F, Al-Ghamdi AA, Al-Ghamdi AA, Aly MM, et al. (2012) A new large-scale synthesis of magnesium oxide nanowires: structural and antibacterial properties. Superlattices Microstruct 52: 200-209.
- Al-Tuwirqi RM, Al-Ghamdi AA, Al-Hazmi F, Alnowaiser F, Al-Ghamdi AA, et al. (2011) Synthesis and physical properties of mixed Co3O4/CoO nanorods by microwave hydrothermal technique. Superlattices Microstruct 50: 437-448.
- Siddiqui AR, Bernstein JM (2010) Chronic wound infection: facts and controversies. Clin Dermatol 28: 519-526.
- Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H (2010) Topical silver for preventing wound infection. Cochrane Database Syst Rev 17: CD006478.
- Aydin S, Ince B, Ince O (2016) Assessment of anaerobic bacterial diversity and its effects on anaerobic system stability and the occurrence of antibiotic resistance genes. Bioresour Technol, 207: 332-338.
- Kumari S, Harjai K, Chhibber S (2010) Evidence to support the therapeutic potential of bacteriophage Kpn5 in burn wound infection caused by Klebsiella pneumoniae in BALB/c mice. J Microbiol Biotechnol 20: 935-941.