Journal of Addiction & Prevention
Download PDF
Review Article
*Address for Correspondence: Barbara B. Oswald, PhD, Department of Psychology, Oxford, OH 45056, Miami University, Tel: (513) 529-6149; Fax: (513) 529-2420; E-mail: oswaldbb@miamioh.edu
Citation: Oswald BB, Corner AC. Rodent Models of Adolescent Alcohol and Drug Self Administration: Implications for Understanding Adult Substance Abuse. J Addiction Prevention. 2013;1(1): 14.
Copyright © 2013 Oswald BB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Addiction & Prevention| ISSN: 2330-2178 | Volume: 1, Issue: 1
Submission: 12 July 2013| Accepted: 13 August 2013 | Published: 16 August 2013
Keywords: Nicotine; Cocaine; Heroin; Rat; Exposure; Brain Development; Addiction
Abbreviations: 5-HT: Serotonin; ACC: Anterior Cingulate Cortex; BAL: Blood Alcohol Level; CPP: Conditioned Place Preference; CPA: Conditioned Place Aversion; CTA: Conditioned Taste Aversion; DA: Dopamine; G: Gram; GABA: Gamma Aminobutyric Acid; ETOH: Ethanol; FR: Fixed Ratio; IC: Intracranial; IG: Intragastric; IP: Intraperitoneal; IV: Intravenous; Kg: Kilogram; LE: Long-Evans Rat; LTD: Long- Term Depression; LTP: Long-Term Potentiation; Mg: Milligram; Mpfc: Medial Prefrontal Cortex; Nacc: Nucleus Accumbens; NMDA: N-Methyl-D-Aspartate; PFC: Prefrontal Cortex; PND: Postnatal Day; PR: Progressive Ratio; SA: Self-Administration; SD: Sprague-Dawley Rat; Sac: Saccharin; Supersac: 125% Saccharin Plus 3% Sucrose Or Glucose
This paper reviews current knowledge of neurobiological and behavioral mechanisms of adolescent drug SA in an effort to elucidate how adolescent drug use may be related to adult substance abuse and dependence. We have chosen to focus primarily on studies investigating SA in rats, as opposed to other methods of drug administration in other species, for two reasons: 1) much is already known about the development of the rat brain during adolescence and there are marked similarities between biochemical changes in rat and human brain during this period, and 2) SA offers an index of volitional drug-taking more akin to adolescent choice behavior than passive drug exposure (i.e., injection or exposure to alcohol vapor). We will also mention results of experiments that have investigated passive drug exposure, but our main focus is to understand the effects of voluntary SA, which may parallel human drug-taking behavior. We will not convey results of research conducted in alcohol-preferring strains of rats, since their genetically selected increased propensity to consume alcohol makes them distinct from the “average” human adolescent. We begin with an overview of behavioral and neurobiological changes during adolescence, to determine how these may increase vulnerability to alcohol and drug use, abuse, and addiction.
Several excellent reviews on adolescent brain development have been published [4-12]. Behaviorally, adolescence in humans and other mammals is marked by increases in impulsivity, desire for novelty, and risk-taking [13,14]. Increases in adolescent risk-taking and impulsivity, coupled with decreases in judgment may be due to differential development of prefrontal, limbic and striatal brain areas that occurs during adolescence [15]. Impulsivity refers to the inability to inhibit inappropriate behaviors, and is controlled by the prefrontal cortex (PFC) [16-20]. The PFC is the part of the brain known to govern “executive control” including higher-order thinking, planning, reasoning, problem-solving, and integrating memories and emotions [21-23]. The PFC is the last area of the brain to develop, and most development of PFC occurs during late adolescence and early adulthood [21,24,25]. As a result, adolescence in mammals is marked by minimal frontal control.
Risk-taking refers to the proclivity to engage in potentially dangerous behaviors, and is likely motivated by an increased desire for novelty [26]. Sensitivity to reward describes the ability to appreciate and approach stimuli of positive valence, while avoiding stimuli of negative valence. Risk-taking, preference for novelty, and sensitivity to reward are regulated by the striatum, a brain area rich with dopaminergic neurons [20,27,28].
In rodents, non-human primates and humans, striatal and limbic development is virtually complete by the onset of adolescence [4,5,7], while the PFC continues to develop into young adulthood [6]. Indeed, although not without criticism (e.g., see [7] for review), Casey and colleagues [4,5,15] suggest that, contrary to popular assumption, adolescent risk-taking is not due to either the lack of behavioral control or increased impulsivity caused by the lack of PFC control, but is instead due to increased novelty seeking and sensitivity to reward, due to the maturation of striatal circuits, in the absence of cognitive control garnered by the frontal cortex. Crews et al. [16,26] have proposed that there is an important evolutionary advantage to these increases in risk-taking, sensation-seeking, and desire for novelty during adolescence. These increases may motivate the adolescent to venture away from their family, to promote mating with genetically diverse partners.
Neurobiologically, the adolescent brain is distinct from both childhood and adult brain [5,15,16,29]. Myelination of cortical neurons increases during adolescence [1], which facilitates communication between the frontal cortex and other cortical and subcortical areas by increasing the speed and efficiency of neural conduction. Juvenile brain maintains multiple connections and undifferentiated brain structures that are absent in the adult brain [12,30,31], and these connections appear to be altered during adolescence via mechanisms of synaptogenesis and pruning. Synaptogenesis increases during early adolescence but is followed by a period of marked decrease or pruning that occurs, conceivably in an effort to strengthen frequently used connections, while discarding unnecessary ones [1,31]. Synaptic pruning likely leads to the decreases in cortical gray matter and brain volume, especially within prefrontal cortex (PFC), observed during adolescence [15,16]. Markham et al. [32] demonstrated that portions along the midline of the PFC known as the medial prefrontal cortex (mPFC) decreased significantly the number of neurons from PND 35 to PND 90, losing an average of 19% in female rats and 5% in male rats. Loss in neural tissue was specific to just the ventral mPFC; the adjacent dorsal PFC and anterior cingulate cortex (ACC) did not experience neural loss during the same time period.
Important changes in neurotransmitter density also occur during adolescence.Dopamine (DA)is a neurotransmitter implicated in reward, risk-taking, identifying novel stimuli, and drug abuse [8,9,33-42]. Several lines of evidence indicate that DA activity peaks during adolescence [2,10,43-49]. DA-containing neurons and the number of postsynaptic DA receptors have been found to increase and then decrease across adolescence [43,46,47]. Benes and colleagues 49] showed transient increases in DA and serotonin (5-HT) fiber density during adolescence that did not extend into adulthood. Interestingly, dopamine D1 and D2 receptors in the striatum were found to peak in early adolescence that were lost by young adulthood [10,44,47] , while D1 and D2 receptors in PFC did not increase until late adolescence or beyond [15]. As noted above, these altered densities in DA receptors between striatal and prefrontal areas could mediate increases in risk taking and sensitivity to reward often seen during adolescence, although concurrent changes in other systems, for example, cannabinoid [15], glutamate, or GABA receptors, may also be responsible. Significant changes occur in both GABA [48,50] and glutamate during adolescence, including alterations in NMDA receptors, which could mediate alterations in synaptic density that occur during adolescence, specifically long-term potentiation (LTP) and long-term depression (LTD; e.g., see [51] and especially [31] for a discussion of LTP and LTD and its relation to synaptogenesis and pruning during adolescence).
In summary, marked differences exist between the juvenile, adolescent, and adult brain. Subcortical structures such as the striatum are nearly fully mature by the onset of adolescence, while the PFC, the brain structure that mediates behavioral inhibition, planning for the future, and “executive control”, continues to develop into young adulthood. Dopamine and glutamate are two neurotransmitter systems that exhibit distinct developmental changes during adolescence, with DA levels peaking and then declining in adolescence, and post-synaptic glutamatergic NMDA receptors also changing density. These neurobiological and neurochemical changes appear to have tremendous significance on reward, risktaking, impulsivity, and learning which profoundly impact adult functioning. These changes further appear to be highly susceptible to environmental influence [10,52-55], which makes adolescence a “sensitive period” of brain development, during which plastic changes occur to motivate long-term behavioral and neurobiological effects [11,31,56].
The effects of alcohol on adolescent brain has been extensively reviewed by others [31,56-60]. Exposure to alcohol during adolescence has been shown to lead to distinct brain changes that typically last through adulthood. In animal models, exposure to binge levels of ethanol (ETOH) of 3 g/kg/day, decreased the number of glial cells in the PFC in adult male but not female rats [61,, and increased cell death in the necortex, hippocampus, and cerebellum [62,. In mice, 5g/kg/day of intragastric (IG) ETOH decreased the number of cholinergic forebrain neurons and brain volumes within the basal forebrain and olfactory bulb [63]. Exposure to ethanol vapor during adolescence in rats affected EEG in adulthood, decreasing the P3 component of the ERP as well as slow-wave sleep [64]. In adolescent rat hippocampal slices, alcohol significantly decreased glutamatergic NMDA synaptic potentials and LTP [57,65]. Exposure to ETOH during adolescence increased extracellular DA volume compared to adults, and also downregulated D2 receptors in PFC [39], in addition to decreasing expression of DA and cholinergic genetic markers[63]. Intermittent exposure to ETOH increased cortical thickness in left frontal pre-temporal and ventral neocortex in rats [66].
In humans, alcohol use during adolescence has been shown to decrease white matter volume, especially along the superior longitudinal fasciculus, a pair of fiber tracts extending from the front to the back of the brain, connecting the four cortical lobes to integrate somatosensory, spatial, and motor information [67]. Another study of 16-19 year old binge drinkers found increased left frontal cortical volume in female binge drinkers, but decreased left frontal volume in males that correlated with detrimental effects on several measures of cognition including attention and inhibition [68]. Adolescent binge drinking decreased volume of the hippocampus [69] and led to distinct alterations in cerebral blood flow (see [58] for review).
Although the exact impact of each of these neurobiological effects on the development of abuse and addiction disorders is unclear, altered sensitivity of adolescent brain, especially brain mechanisms mediating sensation-seeking and reward such as DA, the striatum, and the nucleus accumbens (NAcc), behavioral inhibition by the PFC, and learning via hippocampal function, make adolescents particularly vulnerable to brain alterations that could affect later motivational behaviors, including a propensity to develop dependence and addiction disorders. Future animal research needs to confirm that these brain changes are induced by early exposure to drugs, and are not the result of underlying genetic and environmental predispositions.
Animal models, particularly those using rodents, offer a costeffective means of assessing neurochemical and neurobiological changes during adolescence. Rodent models permit experimental control of genetic, social, and environmental factors that may impact drug-seeking behavior, factors that cannot be easily controlled in human studies. Rodent models facilitate longitudinal investigations of adolescent drug exposure on behavioral, cognitive, and neurobiological functioning during adulthood. However, one challenge with rodent research concerning the effects of drugs during adolescence is that adolescence in the rat is brief, approximately 15 days.
Adolescence is defined as the period just prior to adulthood when changes in hormonal and biological functioning lead to puberty, that is, sexual maturity [2,14,70,71]. In males, sexual maturity is defined as producing viable sperm, and in the rat, this typically begins around postnatal day (PND) 30 and is usually complete by PND 50, although continued physiological changes and sexual maturity may not be fully complete until PND 63 [14]. In female rats, the onset of physiological changes leading to puberty could begin as early as PND 20 [1], with first ovulation typically occurring around PND 38, although a regular estrous cycle may not occur until PND 50 [2,70,71]. Thus the “average” period of adolescence in the rat is typically defined within the 15-day window from PND 28-42, independent of gender [14,72].
Due to the brevity of rat adolescence, it is challenging to develop models to study the effects of alcohol and drugs in this species, especially self-administration (SA). Alcohol SA has therefore been studied most frequently in adolescent rats, since alcohol can be administered orally without requisite surgeries and recovery times, extensive behavioral training, etc. Nonetheless, challenges exist when establishing alcohol SA in rats.
Most strains of laboratory rat do not readily self-administer alcohol, and often find passive alcohol administration aversive [73-77] (but see [78] for exceptions). Despite these challenges, several rodent models of alcohol SA have been developed. One model relies on a number of genetically inbred strains of “alcohol-preferring” rat, that, as the name suggests, readily self-administer intoxicating doses of alcohol. However, genetic susceptibilities that predispose these rats to ingest large amounts of alcohol are likely unique to these animals and do not easily translate to “typical” human use. Therefore, we have chosen to focus on research conducted in non-alcohol preferring outbred strains of laboratory rat, due to their variable propensity to self-administer alcohol, which may be more analogous to humans.
Under limited access conditions where alcohol is only available for short periods of 30-120 minutes a few days each week, nonalcohol preferring rats can be trained to self-administer unsweetened ethanol at concentrations as high as 20% [79]. Sweetening the ETOH solution may lead to faster training [80-82], and can induce intake of concentrations up to 40% [82]. Also, many humans, especially adolescents, choose sweetened alcoholic beverages (i.e., “alcopops”, flavored malt- or wine-based drinks typically sold in single servings and containing roughly 5% alcohol per volume), making sweetened ETOH consumption in the rat a valid model of human adolescent drinking [83,84]. Yet there are important concerns with using sucrose to sweeten ETOH solutions. The addition of sucrose can alter the metabolism of alcohol and decrease overall blood alcohol content (BAC). Thus, in studies comparing sucrose-flavored ETOH to plain ETOH, sucrose-flavored alcohol may be preferentially consumed not because animals prefer the taste, but because sucrose decreases the overall effect of the alcohol [85,86]. Instead of sucrose, some investigators sweeten ETOH with saccharin or “supersac” (a low dose of saccharin combined with a low dose of sucrose or glucose), which may encourage higher alcohol intake while minimizing negative effects on BAC [87-89].
To further simulate human consumption, researchers have evaluated the effects of beer, wine, and even “jello shots,” ETOH dissolved in a gelatin matrix, for promoting SA in animals [90-97]. Perhaps surprisingly, male and female Wistar rats did not prefer a commercially-available Italian white wine diluted from 11% to 10% alcohol content over unflavored 10% ETOH [94,95]. Conversely, studies have found that rodents prefer beer (especially non-alcoholic beer [92,98] over both water and unflavored ETOH [91,92,98-100] . Beer has been used to establish SA in both adolescent and adult rats, although care must be taken to either remove carbonation or use a special clog-free sipper [98,100]. Frequently, commercially available non-alcohol “near beers” are used as the vehicle, and 95% ethanol is added. An alcohol-gelatin matrix akin to an alcoholic “jello shots,” consumed by human adolescents [101] has also been used to encourage SA in adult rats [96-98].
Thus alcohol SA has been reliably established in rodent models. Rats will voluntarily consume behaviorally active doses of ETOH dissolved in water, sweetened ETOH solutions, beer containing 4-20% ETOH per volume, or ETOH dissolved in gelatin. Under limited access conditions, rats consume about 0.5-1 g/kg of ETOH per hour, with most consumed immediately upon access[91,98,102-105]. Under unlimited access conditions, that is, when alcohol is available for 23 or more hours each day, adolescent rats may consume up to 7.5 g/kg/day or more, while adults consume about half, or 2-4 g/ kg/day [79,104,106-108]. Ethanol doses of 0.5 g/kg are intoxicating [88,109] . In the United States, intoxication is defined as having a blood alcohol level (BAL) of 0.08 or above (80 mg of ETOH per 100 mL of blood, or 80 mg%) [110]. BALs greater than 80 mg% cause significant behavioral changes and motor impairment in humans and animals [105]. Self-administration of >0.5 g/kg in the rat can lead to blood alcohol levels between 50-70 mg% [88,105,109], while doses of 2 g/kg lead to BALs of 100 mg% and higher [87,97,111]. Thus SA of behaviorally relevant doses of alcohol can be established in the rat.
A number of laboratories report that adolescent rats consume roughly twice the amount of alcohol as adults [89,91,104,107,112-115], especially sweetened ETOH [88,89,114] or beer [91,100] (see Table 1). Control experiments verify that increased alcohol intake in adolescents is not due to increased caloric needs or higher ingestive behavior that may be related to growth. In contrast, unsweetened ETOH may not be preferred by adolescent rats. A recent study by Blomeyer et al. [107] did not find increased intake of 5% v/v unsweetened ETOH in pubertal (PND 40) male Wistar rats compared to adult (PND 90), as both adolescent and adult rats consumed roughly 0.4 g/kg of ethanol per session. While the 5% volume solution used in that study was chosen to more accurately reflect the concentration of alcohol contained in beer and alcopops consumed by human adolescents, it is at least half of that used in many other studies noted above, although the net intake of alcohol consumed was nearly the same (~0.5 g/kg per session)[88,104,112]. It could be that unsweetened alcohol [85] or lower ETOH concentration [109] led to increased blood alcohol levels, necessitating lower intake, although it is unclear why adolescents did not self-administer more than adults in this study. However, Blomeyer et al. tested only male rats, and several studies [77,88,94,116] note significant gender differences in both adolescent and adult alcohol intake.
adol = adolescent; F = female; LE = Long-Evans strain of rat; M = male; PND = postnatal day; SA = self-administration; sac = saccharin; SD = Sprague-Dawley strain of rat; supersac = 0.125% saccharin plus 3% sucrose orglucose
In non-alcohol preferring lines, female rats and mice have been found to have higher rates of SA at all ages, including adolescence [77,88,94,116]. Broadwater et al. [88] found that female adolescent Sprague-Dawley rats drank more unflavored alcohol (20% ETOH in plain water), more sweetened alcohol (10% ETOH in a supersac solution), and also more sweetened solution alone. Research with other drugs including nicotine and cocaine also report higher levels of intake in adolescent females ([117-122]; discussed below). It is unclear why this occurs, but in humans, adolescent females have also been found to engage in high levels of drug use and binge drinking compared to males, and this number has been steadily on the rise since 1990 [68,83]. Hormones are suspected to motivate these differences, and several studies have assessed the effects of gonadectomy on alcohol intake and preference in male and female rats [76,77]. Vetter- O’Hagen and Spear [123] found that gonadectomy during early adolescence (PND 23) in males increased alcohol SA to female levels, an effect that was reversed by injections of testosterone. Sherrill and colleagues [76] reported that females were less sensitive to the aversive properties of alcohol, and unlike adult males, failed to develop a conditioned taste aversion to flavored water paired with injections of 1.5 g/kg ETOH. Together these findings suggest that testosterone may interact with alcohol to produce aversive effects, although studies are needed to further verify the influence of hormones on the behavioral effects of alcohol.
Does consuming alcohol during adolescence increase intake in adulthood? In humans, it appears that adolescent alcohol misuse can lead to adult abuse and dependence [124-126]. However, human studies on alcohol intake ethically must be correlational in nature - we cannot force random samples of people to drink during adolescence in an effort to assess their adult drinking patterns. Further, adolescent drinkers are a self-selected population who consistently score high on certain behavioral measures including sociability, risk-taking, and sensation-seeking [124,127], factors that may also prompt adult drinking even in the absence of adolescent exposure. Due to the multitude of genetic and environmental factors impacting adolescent alcohol use, it is useful to assess the influence of adolescent alcohol exposure in animals.
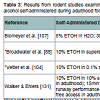
Unfortunately, data from animal studies in outbred lines of non-alcohol preferring rats do not offer a clear understanding of the impact of adolescent alcohol intake on adult proclivity to abuse alcohol. Forced exposure to alcohol during adolescence via either injected bolus or inhaled doses of alcohol vapor may increase alcohol intake in adult animals [39,106108,114,128,129], but not always [82,130] (see Table 2). Studies of volitional SA also lack consensus (see Table 3). For example, Bloymeyer et al. [107] found that SA of 5% ETOH during adolescence increased alcohol intake in adulthood from 0.5g/kg/day to 0.7 g/kg/day, as assessed across 10 days. Broadwater et al. [88] also found that free access to 10% ETOH during adolescence increased SA during adulthood, but this effect was only temporary, and dissipated within 2 weeks. Since Blomeyer and colleagues only assessed adult intake for 10 days, it is not feasible to conclude that increased SA would be sustained through adulthood. Vetter and colleagues [104] did not support increased intake following adolescent SA, noting that even though adolescent Sprague-Dawley rats self-administered significantly more than adults, this effect did not carry over into adulthood. While Criado and Ehlers [106] reported that one week of SA during adolescence followed by 8 weeks of exposure to alcohol vapor increased alcohol SA during adulthood, alcohol intake among the adolescent-exposed rats in this study did not exceed the intake of rats pre-exposed only during adulthood in a previous experiment in this lab [131], making it difficult to declare that exposure during adolescence caused greater adult use.
*Did not find significant increase in adolescent intake
M = male; F = female; SD = Sprague-Dawley strain of rat; LE = Long-Evans strain of rat; sac = saccharin; supersac = 0.125% saccharin plus 3% sucrose or glucose; PND = postnatal day; SA = self-administration; adol = adolescent
Thus, results from rodent studies do not confirm that either exposure to or SA of alcohol during adolescence significantly increases adult intake. However, one difference between these studies may be the time at which alcohol administration first began, that is, early versus late adolescence. As noted above, significant brain changes occur in early adolescence, which could increase subjective feelings of reward, or motivate long-term neural plasticity.
Research in humans has shown that people who engage in experimentation with alcohol and drugs at an early age are at a greater risk for addiction problems as adults [40,84,132]. It has therefore been proposed thatexposure to drugs including alcohol during late childhood and early adolescence can induce long-term changes in the brain to motivate addiction. However, results from animal studies do not confirm a clear relationship between early drug exposure and later adult SA. For example, Alaux-Cantin and colleagues [108] reported that Sprague- Dawley rats repeatedly exposed to binge levels of 3 g/kg injections of alcohol for 14 days during early adolescence (PND 30-43) consumed significantly more alcohol as adults than rats exposed to alcohol during late adolescence (PND 45-58). Criado and Ehlers [106] also reported that alcohol exposure beginning at PND 35 modestly increased adult SA from 0.7 g/kg/day to 1.0 g/kg/day. However, this effect was not different from adult rats in another study who were previously exposed to alcohol vapor as adults [106], tempering enthusiasm for the conclusion that early adolescent exposure increased adult intake. Vetter and colleagues [104] and Broadwater and colleagues [88] allowed rats free access to alcohol during early adolescence at PND 27-28, and even though during adolescence the rats self-administered higher doses of ETOH than adult PND 69-70 rats, the adolescents did not consume greater amounts as adults.
*Did not find significant increases in adolescent intake
M = male; F = female; SD = Sprague-Dawley strain of rat; LE = Long-Evans strain of rat; sac = saccharin; supersac = 0.125% saccharin plus 3% sucrose or glucose; PND = postnatal day; SA = self-administration; adol = adolescent
In summary, while some make the argument that early drug exposure increases the likelihood of later SA, many studies on the effects of alcohol during early adolescence (~PND 25) have found little effect on adult alcohol consumption [39,88,104,114]. This makes it difficult to ascertain that exposure to alcohol during early adolescence induces long-term behavioral and brain changes to cause addiction. In humans, it is more likely that teens who engage in early use of alcohol and drugs are affected by a variety of genetic and environmental factors related to addiction, and that dependence and addiction problems observed in adulthood are not exclusively the result of early exposure to alcohol and/or other drugs. Indeed, early drug use in humans is associated with higher levels of sensation-seeking, conduct and behavioral disorders, low academic performance, low parental involvement, and familial history of addiction [83,127,133], each of which has the potential to motivate abuse and addiction independent of when substance use is initiated. Thus it remains unclear whether adolescent exposure to alcohol leadsto alcohol abuse as an adult. Next we consider research on the effects of other drugs of abuse, to determine whether there may be drugspecific influences that motivate adult dependence.
As illustrated above, evaluating alcohol SA in adolescent rats has posed many challenges. Likewise, assessing SA of other drugs hasnot been easy. For one, most other drugs of abuse do not achieve behaviorally relevant plasma concentrations via oral administration, and it is virtually impossible to get a rat to voluntarily inhale (“smoke”) or insufflate (“snort”) drugs in a manner similar to human consumption. As a result, SA in rodents is typically established by surgically implanting intravenous (IV) catheters, through which small doses of drugs may be infused. Rats can be trained to selfinject drugs by connecting the catheters to syringe pumps connected to operant boxes. Rats can be trained to press levers in the operant box which can mechanically activate the syringe pump to release tiny amounts of drugs into the catheter.
While quite effective for establishing SA, IV drug injection can be problematic, especially in adolescent rats. For one, it requires technical expertise to perform the surgery, and special care must be taken with the smaller veins of adolescents which grow over time. Secondly, it requires post-operative recovery and sometimes lengthy training regimens, the combination of which can exceed the brief 15-day period of adolescence in the rat. Maintaining patency of the catheters is also difficult, especially for longitudinal studies, due to increases in body size that can influence position of the tubing, as well as challenges with ensuring continued functionality (i.e., clogging, infection, etc.). Finally, several drugs that are abused by human adolescents are not readily self-administered by rodents, for example nicotine [134,135] and THC [136,137], the active ingredient in marijuana. As a result, virtually no studies exist testing the effects of marijuana SA in adolescent rats, and research on SA of other drugs during adolescence remains sparse. Literature exists on nicotine, as nicotine, like alcohol, will be self-administered by rats with training [138,139] , and adolescents may experience fewer aversive effects than adults following injections of nicotine [140,141] . Data are also available on the effects of adolescent SA of cocaine and heroin, and so we review results from that research below as well.
Although tobacco consumption is the most preventable cause of death in the United States [140,141], thousands of American teenagers begin smoking every day [142]. Even occasional tobacco use during adolescence can escalate quickly–within days or months– into long-term nicotine addiction [143]. Animal models demonstrate that adolescent rats self-administer higher amounts of nicotine than adults, and initiating nicotine use during adolescence leads to SA of higher doses in adulthood [142]. For example, an early study by Levin and colleagues [118] trained adolescent (PND 32) and adult (PND 62) female rats to self-administer nicotine by first training animals to bar press for food reinforcement for 4 days, followed by 4 days in which bar presses resulted in simultaneous presentation of a food pellet plus an IV injection of nicotine (0.03 mg/kg). For the next 14 days, bar presses yielded nicotine injections alone. During this initial period of SA, adolescents self-infused more nicotine than adults, delivering an average of 0.4 mg/kg of nicotine per session (13 infusions) compared to 0.25 mg/kg (8 infusions) for adults, doses comparable to nicotine intake in humans [118]. Animals were then tested for nicotine SA over the next 4 weeks, and although SA decreased slightly as adulthood approached, the female rats in this study beginning as adolescents continued to self-administer more nicotine, averaging 10 infusions per session ( 0.3 mg/kg) compared to 8 infusions (roughly 0.2 mg/kg) in the adults. Other studies by this group confirm that females continue to self-administer higher levels of nicotine into adulthood [144].
Gender differences in nicotine SA exist. Adolescent males (PND 40-46) self-administered nearly triple the amount of nicotine of adult males, although intake tapered off after 2 weeks as the males approached adulthood (~PND 60), such that adolescent males did not self-administer greater amounts of nicotine as adults [117,144]. Chen and colleagues also reported that in Lewis rats, adolescent females acquired nicotine SA more rapidly than adults, and adolescent exposure caused females to self-administer significantly more nicotine as adults. Other studies in males agree that pre-exposed males do not self-administer more as adults [120], although contrary to the results reported by Levin and colleagues, these studies failed to find higher levels of SA for males during adolescence. Together these findings suggest that nicotine SA can be established in adolescent animals, and adolescents may be prone to self-administer greater amounts of nicotine than adults. Females appear to be more sensitive to the prolonged rewarding effects of nicotine, as they appear to be more likely to continue high levels of nicotine intake into adulthood. Some research suggests that hormonal differences could mediate the rewarding effects of nicotine [145,146], as well as possible gender differences in post-synaptic densities of nicotinic receptors [119,147-149], although future research needs to ascertain the role of each of these on reward and nicotine function.
While many studies have assessed cocaine SA in adult rats, only a few have evaluated SA during adolescence. Unlike studies with alcohol and nicotine, there do not appear to be age differences in the rate at which cocaine SA is acquired nor the total amount of cocaine taken over time, at least not in outbred strains of laboratory rats. Further, evidence suggests that SA during adolescence may decrease cocaine seeking behavior during adulthood. For example, Frantz et al. [150] compared the effects of self-administered and injected cocaine on late adolescent (PND 37-52) and adult male Wistar rats (PND 72- 90). Adolescents had an attenuated response to the motor-stimulating effects of cocaine, suggesting that they may be more tolerant to the negative effects of cocaine and therefore prone to take higher doses. However, adolescent rats did not exhibit higher levels of selfadministration, nor did they show altered levels of dopamine in the NAcc in response to IV (0.37-2.92 mg/kg/infusion) or IP injections (20 mg/kg) of cocaine. Kerstetter and Kantak [151] and Li and Frantz [152] also failed to find significant differences in intake for adolescent over adult rats, although Li and Frantz reported decreased cocaineseekingbehavior in adolescents-turned-adults following a 30-day abstinence period [152]. Lynch [121] noted gender differences in the amount of cocaine self-administered in which adolescent female rats self-administered more cocaine than males, but this study did not compare adolescents with adults of either gender.
Conversely, Wong et al. recently reported that adolescent rats acquired cocaine SA more quickly and with lower doses than adults [153]. Adolescents also exhibited increased DA activity in the VTA of the brain, although this contrasts with what Frantz and colleagues reported for DA levels in the NAcc following cocaine exposure [153]. Anker and Carroll [154] also found that male adolescent Wistar rats self-administered more cocaine than adults, and they exhibited greater levels of cocaine-seeking behavior during extinction, suggesting that adolescents may be more vulnerable to relapse following addiction to cocaine. Another study by this group [122] reported that adolescents are more prone to SA cocaine than adults, and that female adolescents exhibit the greatest vulnerability for addiction as indexed by highest SA rates for cocaine. These authors suggested that failures to find significant differences in cocaine SA between adolescents and adults in previous studies was likely the result of testing doses which were too high (>0.4 mg/kg/infusion) or giving rats insufficient access time (< 2 hours per day). Future studies should continue to assess differences between adolescent and adult response to cocaine, to determine if adolescent SA may lead to increased vulnerability for addiction.
The abuse of opioid pain relievers has escalated in the last 10 years, especially among teens [155-157]. Public awareness has led to a decrease in narcotic prescriptions, but this has fostered a significant increase in the illegal consumption of heroin, which may be easier to obtain and less costly than prescription medications [158-160]. Interestingly, animal studies generally report that adolescent rats and mice self-administer lower amounts of several opioids including morphine, oxycodone, and heroin [161-164]. It is unclear why this is, but it has been suggested that adolescents experience greater levels of reward from opiates than adults, possibly due to increased levels of midbrain dopamine that occurs during adolescence [161,162]. A recent study by Doherty and Frantz [164] did find that adolescent rats (PND 35-49) self-administered more heroin than adults (PND 82- 101) at 2 doses (0.025 and 0.05 mg/kg/infusion). However, there were minimal differences between adolescent and adults in the rewarding effects of the drug as measured by breakpoints on a progressive ratio schedule, and adolescents did not exhibit higher levels of drugseeking behavior following withdrawal. These findings suggest that younger rats could be less vulnerable to the reinforcing effects of opiate drugs. Even so, these rodent findings do not match what is generally found with human abusers, who exhibit greater escalation in drug use during adolescence than adulthood [157]. Thus there may be important social or environmental factors mediating opiate use and abuse in humans that need to be further investigated. Future studies should also focus on elucidating age-related differences in endorphin function that may alter abuse liability in adolescent rats.
While not all studies agree, there is ample evidence from humans, non-human primates, and rodents to conclude that adolescents seek out and ingest higher amounts of rewarding stimuli including alcohol, drugs, sweetened water, and the opportunity to engage in social interaction [70,83,88,102,104,118,133,154]. As a result, adolescents may experience greater subjective feelings of reward than adults which, in turn, may encourage higher levels of engagement with reinforcers. Research on the ontogeny of the brain reward system demonstrates that adolescence is a period of idiosyncratic peaks in DA concentration concomitant with the development of striatal circuits governing sensitivity to reward (e.g., [7,20,26,46], see also [6,10,15] for reviews). Yet it is unknown whether these neurobiological substrates correspond to increases in subjective reward sensitivity. Conversely, adolescents may experience decreased reward and intake higher amounts of alcohol and other drugs to compensate for a decline in euphoria. Alternatively, adolescents might feel similar reward valence, but experience decreased negative effects from alcohol and other drugs. For instance, high doses of alcohol induce nausea, motor impairment, memory loss, and a “hangover” effect, while stimulant drugs including cocaine and nicotine produce unpleasant tachycardia and motor hyperactivity. Decreasing these negative side effects could encourage adolescents to ingest higher doses. Research supports each of these hypotheses. For example, as reviewed by Doremus-Fitzwater, Varlinskaya and Spear [13] and Schramm-Sapyta and colleagues [132] , studies utilizing the conditioned place preference (CPP) paradigm demonstrate that adolescents spend more time in places associated with alcohol, nicotine, cocaine and social interaction, suggesting that adolescents experience greater reward or positive valence from these reinforcers. Similarly, tests of conditioned place aversion (CPA) and conditioned taste aversion (CTA) as indices of negative effects have found that adolescents express less aversion to high doses of nicotine and alcohol than adults, as adolescents are less prone to avoid places or flavors paired with these stimuli (see [13,132] for reviews). However, it is difficult to conclude on the basis of CPP or aversion studies that approach or avoidance is due exclusively to the reward valence of stimuli, as a number of other motivational, motor, and behavioral factors may influence approach and avoidance behaviors.
To assess the rewarding properties of stimuli, it is useful to determine the amount of effort an organism is willing to exert to obtain a reinforcer. Indeed, a hallmark of human addiction is the devotion of time, energy, and resources to obtain the addictive substance to the exclusion of other activities. It is assumed that compulsive behaviors are due to do a dysfunction in the brain reward system, such that alternative activities no longer carry reinforcing value. In animals, it is possible to assess reward value or at least motivation to obtain a substance by measuring “response cost” with operant schedules of reinforcement. For example, low fixed-ratio (FR) schedules like FR 1 or FR 2 have low response cost; the organism needs to exert little effort, merely one (FR 1) or two (FR 2) responses, to obtain access to a reinforcer. Increasing the FR requirement increases the cost accordingly, so that higher FR schedules can be used to determine what “price” animals are willing to pay in order to gain access to the reward. Another way to assess reward or motivation is to determine “breakpoint” on progressive ratio (PR) schedules of reinforcement. In PR schedules, increasingly higher numbers of responses are required to obtain a reinforcer, and the “breakpoint” is the point at which animals stop responding. Breakpoints offer an index of reward where higher breakpoints reflect higher reward value, as animals are willing to exert more effort to obtain the same reinforcer. PR schedules may be arranged in a number of ways. One simple method uses a fixed number of additive responses, for example PR 5 or PR 10, where response value must increase on each subsequent trial by N + PR to obtain additional reinforcers.
It has been challenging to assess FR or PR effects for alcohol in outbred strains of rats due to the trouble with establishing operant alcohol self-administration. Nevertheless, Rowland and colleagues [97] developed a unique PR design using access to a polyglucose gelatin matrix as the reinforcer. Adult male Sprague-Dawley rats were trained on an FR 1 schedule of reinforcement where lever presses resulted in access to 0.28 g of gelatin or gelatin plus 10% ETOH. The FR schedule was gradually increased to FR 30 over a period of three months, and then switched to a PR schedule. ETOH-infused gelatin was not more rewarding than gelatin alone, as determined by similar breakpoints to both reinforcers. However, intermittent forced exposure to an intoxicating dose of ETOH (3 g/kg, IP) later increased breakpoints on a PR 10 schedule from 28 to ~38 lever presses for ETOH-containing gelatin. These findings suggest that intermittent exposure to high doses of alcohol (perhaps similar to binge drinking in humans) during adulthood can increase the rewarding value of alcohol. In adolescents, Alaux-Cantin et al. [108] found that pre-exposure to 8 intoxicating doses of ETOH (3 g/kg, IP) increased breakpoints to gain access to 0.1 ml of 10% ETOH. However, these same rats did not show preference for alcohol in a CPP test following either 0.5 or 1.5 g/kg ETOH, making it difficult to conclude that adolescents found alcohol more rewarding. Future studies should continue to compare breakpoints and response cost between adolescents and adults self-administering alcohol.
A few studies have assessed reward valence of nicotine in adolescent versus adult rats using FR and PR schedules [120,144,165]. In contrast to findings from CPP studies, which generally report that adolescents demonstrate preference for places associated with nicotine (see [132] for review), adolescent rats seem to experience less reward from nicotine than adults when assessed with operant measures. For example, Shram and colleagues [165] found that adolescent rats self-administered significantly less IV nicotine than adults at doses of 0.015 and 0.03 mg/kg/infusion, and under a PR schedule of reinforcement, adolescents displayed breakpoints nearly half that of adults. Further, adolescents were less willing to respond for nicotine infusions when the response cost was increased slightly from FR 2 to FR 5 [165]. Sharp and colleagues also reported a decrease in responding for 0.03 mg/kg/infusion of IV nicotine when “price” was increased from FR 1 or FR 3 to FR 5 or FR 7 [120]. Levin et al. [144] found that when switched from a high-cost FR 8 to a less costly FR 1 schedule of reinforcement, adolescent rats continued to respond at a high rate, increasing nicotine intake nearly 8-fold. The authors inferred that adolescents experienced less reward from nicotine since they continued to self-infuse high doses [144], although there are alternative interpretations to this, including decreased aversive effects, or a lack of behavioral inhibition that motivated the high rates of response in these adolescents.
Only a few studies have assessed the rewarding properties of cocaine during adolescence with operant measures. Wong et al. [153] found that post-pubertal male adolescent Sprague-Dawley rats (PND 42) self-administered significantly more IV cocaine than either prepubertal (PND 35) or adult rats (PND 88) at doses ranging from 0.15- 0.60 mg/kg/infusion. Moreover, adolescents demonstrated increased motivation to obtain cocaine compared to adults, as they continued to respond for cocaine infusions even as price increased from FR 3 to FR 24. Insofar as motivation to bar press for cocaine infusions gauges reward, these results suggest that adolescents find cocaine more rewarding. In an effort to determine whether this increase in subjective reward was mediated by brain reward pathways, Wong and colleagues [153] hypothesized that the increased density and firing rate of DA-containing cells within the VTA (described above) motivates higher SA rates during adolescence. If true, then decreasing DA firing in adolescent rats should lower SA rates to adult levels, and increasing DA firing in adults should raise SA rates to that of adolescents. To decrease DA firing rates, Wong et al. gave IP injections of 0.2 mg/ kg of the D2 autoreceptor agonist quinpirole, a dose found to inhibit DA cell bodies without having appreciable effects on D2 receptors directly. As expected, decreasing DA firing rates with quinpirole decreased adolescent SA rates to adult levels, while increasing DA firing rates with the D2 antagonist eticlopride caused adult rats to increase their SA to match adolescents. Collectively, these results suggest that adolescents derive greater reward from cocaine, an effect which is mediated by increased levels of DA within the VTA.
In studies with heroin, Doherty and Frantz [164] found that adolescents self-administered more drug when cost was low (FR 1 to FR 5 schedules of reinforcement). Yet when price was increased on a PR schedule of reinforcement, adolescents did not exhibit differences in breakpoints from adults. These results suggested that adolescents were not willing to exert more effort to obtain the drug. On the basis of this limited data, it does not appear that adolescents experience greater reward from heroin and possibly other opiates, although future research must verify these results.
Based on these studies, it appears that adolescents may experience greater reward from DA agonists like cocaine that directly increase synaptic concentrations of DA. Dopamine agonists may enhance reward during adolescence due to their ability to stimulate the higher number of DA neurons within the striatal reward pathway. On the other hand, drugs with indirect actions on DA such as alcohol, nicotine and heroin do not appear to increase subjective feelings of reward, and in the case of nicotine, may actually decrease reward valence during adolescence. Attenuated reward following non-DA drugs may be the result of downstream reductions in synaptic DA or downregulation of D2 receptors, which have been found to occur following adolescent exposure to nicotine and alcohol [39,166,167]. However, this research is in its infancy and additional studies are necessary before firm conclusions may be drawn. Future studies should continue to test the hypothesis that directly enhancing DA can increase reward during adolescence. The rewarding effects of other DA agonists including amphetamine and methamphetamine, and perhaps non-abused DA agonists such as nomifensine, should be examined more carefully in adolescent populations. Studies should also directly compare the reward value of dopaminergic and non- DA drugs of abuse in adolescents, in an effort to determine whether stimulating DA during adolescence can increase subjective feelings of reward and possibly motivate addiction.
Research in humans suggests that high levels of adolescent drug use can lead to abuse and addiction in adulthood, but the reasons why this occurs are unclear. Is exposure to alcohol and other drugs during adolescence enough to promote long-lasting brain changes that lead to adult addiction disorders? Or do genetic predispositions motivate both adolescent experimentation and later adult addiction? These questions are difficult to answer in human studies, since genetic and environmental factors cannot easily be teased apart due to ethical constraints. Animal investigations of the effects of alcohol and other drugs during adolescence remain critical for understanding brain and behavioral vulnerabilities that lead to excessive misuse and abuse in adulthood.
While several laboratories corroborate that adolescent rats are prone to voluntarily ingest greater amounts of alcohol, nicotine, cocaine, and heroin than adults [100,104,112,117,118,140,153,154,164,168,169] , the impact of adolescent pre-exposure on adult drug use has not been fully elucidated. Some studies of alcohol use report increased adult intake following adolescent exposure (both passive exposure and SA) [75,87,106-108,131,170] , but others do not [39,82,88,91,104,130] . Studies with nicotine generally report that adolescent SA increases adult intake in female rats [117,118,165], although at least 2 studies have not found an impact of adolescent SA in male rats [165,169]. Adolescent rats do not always prefer to selfadminister cocaine [150,152], and adolescent pre-exposure does not appear to increase motivation to take cocaine or heroin as an adult in rats [152,164]. These findings make it difficult to ascertain that drug exposure during adolescence modifies brain reward signaling to increase addiction potential.
Instead, it is well documented that adolescents are more prone to risk-taking and preference for novelty than adults, which is perhaps a biologically adaptive mechanism to promote greater diversity in mate selection [11,26]. Risk-taking, sensation-seeking, and preference for novelty motivate both humans and animals to seek out new experiences, which may be relevant to drug SA. Studies have shown that rats self-administering high doses of alcohol [91,104] and nicotine [117] during adolescence decreased their intake significantly by young adulthood. This pattern of alcohol and drug use mimics that of human teens, who often escalate drug intake during high school and college, but taper use in early adulthood (after age 23) [83,84,124]. In humans, decreased consumption in adulthood hasbeen attributed to competition between family and work obligations that leave less time to indulge in alcohol and drug use. However, evidence from rodent studies demonstrate that rats also decrease alcohol and nicotine intake in adulthood, independent of increased responsibility associated with human development. These findings suggest that there are distinct behavioral and/or neurobiological changes in adulthood that prompt decreased drug intake. Future research must strive to determine the exact underpinnings of this decline in substance abuse observed in adulthood, as understanding these mechanisms could lead to the development of treatments to temper substance abuse during adolescence.
Yet there remains a subpopulation of young adolescents who engage in extremely risky alcohol and drug use very early in life, and these teens appear at greatest risk for later abuse and addiction problems. Based on animal research, there does not appear to be a causal mechanism between exposing the brain to alcohol and drugs in early adolescence and later addiction. Instead, it appears that there are other genetic and environmental factors influencing both the tendency to experiment with drugs early in life and later addiction, such as a higher propensity for sensation-seeking and risk-taking, a lack of parental involvement, family history of addiction problems, etc. [83,133,171]. Future research in animals can help to elucidate these factors. For example, there is a high level of individual difference in the amount of alcohol intake among rats. Future studies should focus on comparing behavioral and neurobiological differences between adolescent “high-users” and “low users.” Pre-exposure assessment should also occur, in an effort to determine whether brain differences are present prior to the onset of SA, or if certain organisms appear more susceptible to permanent neurobiological changes following drug exposure that could motivate long-term addiction problems.
Rodent Models of Adolescent Alcohol and Drug Self- Administration: Implications for Understanding Adult Substance Abuse
Barbara B. Oswald*and Alexandra C. Corner
- Department of Psychology, Oxford, OH 45056, Miami University, USA
*Address for Correspondence: Barbara B. Oswald, PhD, Department of Psychology, Oxford, OH 45056, Miami University, Tel: (513) 529-6149; Fax: (513) 529-2420; E-mail: oswaldbb@miamioh.edu
Citation: Oswald BB, Corner AC. Rodent Models of Adolescent Alcohol and Drug Self Administration: Implications for Understanding Adult Substance Abuse. J Addiction Prevention. 2013;1(1): 14.
Copyright © 2013 Oswald BB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Addiction & Prevention| ISSN: 2330-2178 | Volume: 1, Issue: 1
Submission: 12 July 2013| Accepted: 13 August 2013 | Published: 16 August 2013
Abstract
In humans, experimentation with drugs (including alcohol) typically begins in adolescence. Adolescent experimentation can escalate to abuse and dependence in adulthood. Converging evidence from molecular, cellular and systems analyses demonstrate adolescence as a plastic period of brain development when important modifications occur in reward pathway signaling. Environmental factors, including exposure to drugs, have the potential to impact these critical neurodevelopmental changes. Rodent models offer well-controlled and cost-effective methods of assessing neurobiological and longitudinal effects. This paper reviews research on drug self-administration during adolescence and subsequent propensity for abuse in adulthood as determined by rodent models. Self-administration studies report increased drug intake in adolescent rats, independent of changes in ingestive behavior. Some longitudinal studies report that adolescent drug use leads to increased consumption during adulthood, but results are variable. Behavioral tests following alcohol and nicotine report attenuated negative effects such as motor impairment, and increased positive effects such as association with reward in adolescents. Studies of “response cost” suggest that adolescents may experience greater subjective reward from direct dopamine agonists like cocaine, but may perceive lower levels of reward from non-dopaminergic drugs like alcohol, nicotine, and heroin. Collectively, research demonstrates that drug abuse spikes during adolescence in humans and rodents, and this may be due to increases in risk-taking and sensation-seeking which characterize adolescence in these species. However, drug use declines significantly through early adulthood in most organisms, with only certain “high risk” populations engaging in continued abuse and addiction as adults. Future studies should focus on elucidating 1) neurobiological differences between high and low adolescent users, and 2) the neurobiological underpinnings of the decrease in drug use that consistently occurs during adulthood, in an effort to discover possible identification, prevention, and treatment measures for the subpopulation of adolescents who exhibit abuse and addiction disorders into adulthood.Keywords: Nicotine; Cocaine; Heroin; Rat; Exposure; Brain Development; Addiction
Abbreviations: 5-HT: Serotonin; ACC: Anterior Cingulate Cortex; BAL: Blood Alcohol Level; CPP: Conditioned Place Preference; CPA: Conditioned Place Aversion; CTA: Conditioned Taste Aversion; DA: Dopamine; G: Gram; GABA: Gamma Aminobutyric Acid; ETOH: Ethanol; FR: Fixed Ratio; IC: Intracranial; IG: Intragastric; IP: Intraperitoneal; IV: Intravenous; Kg: Kilogram; LE: Long-Evans Rat; LTD: Long- Term Depression; LTP: Long-Term Potentiation; Mg: Milligram; Mpfc: Medial Prefrontal Cortex; Nacc: Nucleus Accumbens; NMDA: N-Methyl-D-Aspartate; PFC: Prefrontal Cortex; PND: Postnatal Day; PR: Progressive Ratio; SA: Self-Administration; SD: Sprague-Dawley Rat; Sac: Saccharin; Supersac: 125% Saccharin Plus 3% Sucrose Or Glucose
Introduction
Research on the effects of exposure to alcohol and other drugs during adolescence has increased dramatically over the last 20 years [1-3]. This research has fostered an increased understanding of neurobiological and behavioral mechanisms underlying adolescent drug self-administration (SA), but much remains to be learned regarding cellular, molecular, and behavioral changes that link adolescent drug use with adult substance abuse and dependence. Understanding these mechanisms is critical for the development of successful treatment and prevention programs.This paper reviews current knowledge of neurobiological and behavioral mechanisms of adolescent drug SA in an effort to elucidate how adolescent drug use may be related to adult substance abuse and dependence. We have chosen to focus primarily on studies investigating SA in rats, as opposed to other methods of drug administration in other species, for two reasons: 1) much is already known about the development of the rat brain during adolescence and there are marked similarities between biochemical changes in rat and human brain during this period, and 2) SA offers an index of volitional drug-taking more akin to adolescent choice behavior than passive drug exposure (i.e., injection or exposure to alcohol vapor). We will also mention results of experiments that have investigated passive drug exposure, but our main focus is to understand the effects of voluntary SA, which may parallel human drug-taking behavior. We will not convey results of research conducted in alcohol-preferring strains of rats, since their genetically selected increased propensity to consume alcohol makes them distinct from the “average” human adolescent. We begin with an overview of behavioral and neurobiological changes during adolescence, to determine how these may increase vulnerability to alcohol and drug use, abuse, and addiction.
Adolescent Behavior and Neurobiology of the Adolescent Brain
Risk-taking refers to the proclivity to engage in potentially dangerous behaviors, and is likely motivated by an increased desire for novelty [26]. Sensitivity to reward describes the ability to appreciate and approach stimuli of positive valence, while avoiding stimuli of negative valence. Risk-taking, preference for novelty, and sensitivity to reward are regulated by the striatum, a brain area rich with dopaminergic neurons [20,27,28].
In rodents, non-human primates and humans, striatal and limbic development is virtually complete by the onset of adolescence [4,5,7], while the PFC continues to develop into young adulthood [6]. Indeed, although not without criticism (e.g., see [7] for review), Casey and colleagues [4,5,15] suggest that, contrary to popular assumption, adolescent risk-taking is not due to either the lack of behavioral control or increased impulsivity caused by the lack of PFC control, but is instead due to increased novelty seeking and sensitivity to reward, due to the maturation of striatal circuits, in the absence of cognitive control garnered by the frontal cortex. Crews et al. [16,26] have proposed that there is an important evolutionary advantage to these increases in risk-taking, sensation-seeking, and desire for novelty during adolescence. These increases may motivate the adolescent to venture away from their family, to promote mating with genetically diverse partners.
Neurobiologically, the adolescent brain is distinct from both childhood and adult brain [5,15,16,29]. Myelination of cortical neurons increases during adolescence [1], which facilitates communication between the frontal cortex and other cortical and subcortical areas by increasing the speed and efficiency of neural conduction. Juvenile brain maintains multiple connections and undifferentiated brain structures that are absent in the adult brain [12,30,31], and these connections appear to be altered during adolescence via mechanisms of synaptogenesis and pruning. Synaptogenesis increases during early adolescence but is followed by a period of marked decrease or pruning that occurs, conceivably in an effort to strengthen frequently used connections, while discarding unnecessary ones [1,31]. Synaptic pruning likely leads to the decreases in cortical gray matter and brain volume, especially within prefrontal cortex (PFC), observed during adolescence [15,16]. Markham et al. [32] demonstrated that portions along the midline of the PFC known as the medial prefrontal cortex (mPFC) decreased significantly the number of neurons from PND 35 to PND 90, losing an average of 19% in female rats and 5% in male rats. Loss in neural tissue was specific to just the ventral mPFC; the adjacent dorsal PFC and anterior cingulate cortex (ACC) did not experience neural loss during the same time period.
Important changes in neurotransmitter density also occur during adolescence.Dopamine (DA)is a neurotransmitter implicated in reward, risk-taking, identifying novel stimuli, and drug abuse [8,9,33-42]. Several lines of evidence indicate that DA activity peaks during adolescence [2,10,43-49]. DA-containing neurons and the number of postsynaptic DA receptors have been found to increase and then decrease across adolescence [43,46,47]. Benes and colleagues 49] showed transient increases in DA and serotonin (5-HT) fiber density during adolescence that did not extend into adulthood. Interestingly, dopamine D1 and D2 receptors in the striatum were found to peak in early adolescence that were lost by young adulthood [10,44,47] , while D1 and D2 receptors in PFC did not increase until late adolescence or beyond [15]. As noted above, these altered densities in DA receptors between striatal and prefrontal areas could mediate increases in risk taking and sensitivity to reward often seen during adolescence, although concurrent changes in other systems, for example, cannabinoid [15], glutamate, or GABA receptors, may also be responsible. Significant changes occur in both GABA [48,50] and glutamate during adolescence, including alterations in NMDA receptors, which could mediate alterations in synaptic density that occur during adolescence, specifically long-term potentiation (LTP) and long-term depression (LTD; e.g., see [51] and especially [31] for a discussion of LTP and LTD and its relation to synaptogenesis and pruning during adolescence).
In summary, marked differences exist between the juvenile, adolescent, and adult brain. Subcortical structures such as the striatum are nearly fully mature by the onset of adolescence, while the PFC, the brain structure that mediates behavioral inhibition, planning for the future, and “executive control”, continues to develop into young adulthood. Dopamine and glutamate are two neurotransmitter systems that exhibit distinct developmental changes during adolescence, with DA levels peaking and then declining in adolescence, and post-synaptic glutamatergic NMDA receptors also changing density. These neurobiological and neurochemical changes appear to have tremendous significance on reward, risktaking, impulsivity, and learning which profoundly impact adult functioning. These changes further appear to be highly susceptible to environmental influence [10,52-55], which makes adolescence a “sensitive period” of brain development, during which plastic changes occur to motivate long-term behavioral and neurobiological effects [11,31,56].
Neurobiological Changes Following Adolescent Alcohol Exposure
In humans, alcohol use during adolescence has been shown to decrease white matter volume, especially along the superior longitudinal fasciculus, a pair of fiber tracts extending from the front to the back of the brain, connecting the four cortical lobes to integrate somatosensory, spatial, and motor information [67]. Another study of 16-19 year old binge drinkers found increased left frontal cortical volume in female binge drinkers, but decreased left frontal volume in males that correlated with detrimental effects on several measures of cognition including attention and inhibition [68]. Adolescent binge drinking decreased volume of the hippocampus [69] and led to distinct alterations in cerebral blood flow (see [58] for review).
Although the exact impact of each of these neurobiological effects on the development of abuse and addiction disorders is unclear, altered sensitivity of adolescent brain, especially brain mechanisms mediating sensation-seeking and reward such as DA, the striatum, and the nucleus accumbens (NAcc), behavioral inhibition by the PFC, and learning via hippocampal function, make adolescents particularly vulnerable to brain alterations that could affect later motivational behaviors, including a propensity to develop dependence and addiction disorders. Future animal research needs to confirm that these brain changes are induced by early exposure to drugs, and are not the result of underlying genetic and environmental predispositions.
The Effects of Drug Self-Administration during Adolescence: Adolescence in the Rat
Adolescence is defined as the period just prior to adulthood when changes in hormonal and biological functioning lead to puberty, that is, sexual maturity [2,14,70,71]. In males, sexual maturity is defined as producing viable sperm, and in the rat, this typically begins around postnatal day (PND) 30 and is usually complete by PND 50, although continued physiological changes and sexual maturity may not be fully complete until PND 63 [14]. In female rats, the onset of physiological changes leading to puberty could begin as early as PND 20 [1], with first ovulation typically occurring around PND 38, although a regular estrous cycle may not occur until PND 50 [2,70,71]. Thus the “average” period of adolescence in the rat is typically defined within the 15-day window from PND 28-42, independent of gender [14,72].
Due to the brevity of rat adolescence, it is challenging to develop models to study the effects of alcohol and drugs in this species, especially self-administration (SA). Alcohol SA has therefore been studied most frequently in adolescent rats, since alcohol can be administered orally without requisite surgeries and recovery times, extensive behavioral training, etc. Nonetheless, challenges exist when establishing alcohol SA in rats.
Alcohol Self-Administration in Rats
Under limited access conditions where alcohol is only available for short periods of 30-120 minutes a few days each week, nonalcohol preferring rats can be trained to self-administer unsweetened ethanol at concentrations as high as 20% [79]. Sweetening the ETOH solution may lead to faster training [80-82], and can induce intake of concentrations up to 40% [82]. Also, many humans, especially adolescents, choose sweetened alcoholic beverages (i.e., “alcopops”, flavored malt- or wine-based drinks typically sold in single servings and containing roughly 5% alcohol per volume), making sweetened ETOH consumption in the rat a valid model of human adolescent drinking [83,84]. Yet there are important concerns with using sucrose to sweeten ETOH solutions. The addition of sucrose can alter the metabolism of alcohol and decrease overall blood alcohol content (BAC). Thus, in studies comparing sucrose-flavored ETOH to plain ETOH, sucrose-flavored alcohol may be preferentially consumed not because animals prefer the taste, but because sucrose decreases the overall effect of the alcohol [85,86]. Instead of sucrose, some investigators sweeten ETOH with saccharin or “supersac” (a low dose of saccharin combined with a low dose of sucrose or glucose), which may encourage higher alcohol intake while minimizing negative effects on BAC [87-89].
To further simulate human consumption, researchers have evaluated the effects of beer, wine, and even “jello shots,” ETOH dissolved in a gelatin matrix, for promoting SA in animals [90-97]. Perhaps surprisingly, male and female Wistar rats did not prefer a commercially-available Italian white wine diluted from 11% to 10% alcohol content over unflavored 10% ETOH [94,95]. Conversely, studies have found that rodents prefer beer (especially non-alcoholic beer [92,98] over both water and unflavored ETOH [91,92,98-100] . Beer has been used to establish SA in both adolescent and adult rats, although care must be taken to either remove carbonation or use a special clog-free sipper [98,100]. Frequently, commercially available non-alcohol “near beers” are used as the vehicle, and 95% ethanol is added. An alcohol-gelatin matrix akin to an alcoholic “jello shots,” consumed by human adolescents [101] has also been used to encourage SA in adult rats [96-98].
Thus alcohol SA has been reliably established in rodent models. Rats will voluntarily consume behaviorally active doses of ETOH dissolved in water, sweetened ETOH solutions, beer containing 4-20% ETOH per volume, or ETOH dissolved in gelatin. Under limited access conditions, rats consume about 0.5-1 g/kg of ETOH per hour, with most consumed immediately upon access[91,98,102-105]. Under unlimited access conditions, that is, when alcohol is available for 23 or more hours each day, adolescent rats may consume up to 7.5 g/kg/day or more, while adults consume about half, or 2-4 g/ kg/day [79,104,106-108]. Ethanol doses of 0.5 g/kg are intoxicating [88,109] . In the United States, intoxication is defined as having a blood alcohol level (BAL) of 0.08 or above (80 mg of ETOH per 100 mL of blood, or 80 mg%) [110]. BALs greater than 80 mg% cause significant behavioral changes and motor impairment in humans and animals [105]. Self-administration of >0.5 g/kg in the rat can lead to blood alcohol levels between 50-70 mg% [88,105,109], while doses of 2 g/kg lead to BALs of 100 mg% and higher [87,97,111]. Thus SA of behaviorally relevant doses of alcohol can be established in the rat.
Self-Administration of Alcohol in Adolescent Rats
*Did not find significant increases in adolescent intake
adol = adolescent; F = female; LE = Long-Evans strain of rat; M = male; PND = postnatal day; SA = self-administration; sac = saccharin; SD = Sprague-Dawley strain of rat; supersac = 0.125% saccharin plus 3% sucrose orglucose
Gender Differences in Alcohol Self-Administration
Effects of Adolescent Alcohol Self-Administration on Adult Alcohol Use
Unfortunately, data from animal studies in outbred lines of non-alcohol preferring rats do not offer a clear understanding of the impact of adolescent alcohol intake on adult proclivity to abuse alcohol. Forced exposure to alcohol during adolescence via either injected bolus or inhaled doses of alcohol vapor may increase alcohol intake in adult animals [39,106108,114,128,129], but not always [82,130] (see Table 2). Studies of volitional SA also lack consensus (see Table 3). For example, Bloymeyer et al. [107] found that SA of 5% ETOH during adolescence increased alcohol intake in adulthood from 0.5g/kg/day to 0.7 g/kg/day, as assessed across 10 days. Broadwater et al. [88] also found that free access to 10% ETOH during adolescence increased SA during adulthood, but this effect was only temporary, and dissipated within 2 weeks. Since Blomeyer and colleagues only assessed adult intake for 10 days, it is not feasible to conclude that increased SA would be sustained through adulthood. Vetter and colleagues [104] did not support increased intake following adolescent SA, noting that even though adolescent Sprague-Dawley rats self-administered significantly more than adults, this effect did not carry over into adulthood. While Criado and Ehlers [106] reported that one week of SA during adolescence followed by 8 weeks of exposure to alcohol vapor increased alcohol SA during adulthood, alcohol intake among the adolescent-exposed rats in this study did not exceed the intake of rats pre-exposed only during adulthood in a previous experiment in this lab [131], making it difficult to declare that exposure during adolescence caused greater adult use.
*Did not find significant increase in adolescent intake
M = male; F = female; SD = Sprague-Dawley strain of rat; LE = Long-Evans strain of rat; sac = saccharin; supersac = 0.125% saccharin plus 3% sucrose or glucose; PND = postnatal day; SA = self-administration; adol = adolescent
Thus, results from rodent studies do not confirm that either exposure to or SA of alcohol during adolescence significantly increases adult intake. However, one difference between these studies may be the time at which alcohol administration first began, that is, early versus late adolescence. As noted above, significant brain changes occur in early adolescence, which could increase subjective feelings of reward, or motivate long-term neural plasticity.
Research in humans has shown that people who engage in experimentation with alcohol and drugs at an early age are at a greater risk for addiction problems as adults [40,84,132]. It has therefore been proposed thatexposure to drugs including alcohol during late childhood and early adolescence can induce long-term changes in the brain to motivate addiction. However, results from animal studies do not confirm a clear relationship between early drug exposure and later adult SA. For example, Alaux-Cantin and colleagues [108] reported that Sprague- Dawley rats repeatedly exposed to binge levels of 3 g/kg injections of alcohol for 14 days during early adolescence (PND 30-43) consumed significantly more alcohol as adults than rats exposed to alcohol during late adolescence (PND 45-58). Criado and Ehlers [106] also reported that alcohol exposure beginning at PND 35 modestly increased adult SA from 0.7 g/kg/day to 1.0 g/kg/day. However, this effect was not different from adult rats in another study who were previously exposed to alcohol vapor as adults [106], tempering enthusiasm for the conclusion that early adolescent exposure increased adult intake. Vetter and colleagues [104] and Broadwater and colleagues [88] allowed rats free access to alcohol during early adolescence at PND 27-28, and even though during adolescence the rats self-administered higher doses of ETOH than adult PND 69-70 rats, the adolescents did not consume greater amounts as adults.
Table 3: Results from rodent studies examining self-administration of alcohol during adolescence on adult alcohol self-administration. Results reflect the dose of alcohol self-administered during adulthood for rats previously self-administering alcohol during adolescence (top) or not (bottom), or other groups as noted.
*Did not find significant increases in adolescent intake
M = male; F = female; SD = Sprague-Dawley strain of rat; LE = Long-Evans strain of rat; sac = saccharin; supersac = 0.125% saccharin plus 3% sucrose or glucose; PND = postnatal day; SA = self-administration; adol = adolescent
In summary, while some make the argument that early drug exposure increases the likelihood of later SA, many studies on the effects of alcohol during early adolescence (~PND 25) have found little effect on adult alcohol consumption [39,88,104,114]. This makes it difficult to ascertain that exposure to alcohol during early adolescence induces long-term behavioral and brain changes to cause addiction. In humans, it is more likely that teens who engage in early use of alcohol and drugs are affected by a variety of genetic and environmental factors related to addiction, and that dependence and addiction problems observed in adulthood are not exclusively the result of early exposure to alcohol and/or other drugs. Indeed, early drug use in humans is associated with higher levels of sensation-seeking, conduct and behavioral disorders, low academic performance, low parental involvement, and familial history of addiction [83,127,133], each of which has the potential to motivate abuse and addiction independent of when substance use is initiated. Thus it remains unclear whether adolescent exposure to alcohol leadsto alcohol abuse as an adult. Next we consider research on the effects of other drugs of abuse, to determine whether there may be drugspecific influences that motivate adult dependence.
Self-Administration of Other Drugs in Adolescent Rats
While quite effective for establishing SA, IV drug injection can be problematic, especially in adolescent rats. For one, it requires technical expertise to perform the surgery, and special care must be taken with the smaller veins of adolescents which grow over time. Secondly, it requires post-operative recovery and sometimes lengthy training regimens, the combination of which can exceed the brief 15-day period of adolescence in the rat. Maintaining patency of the catheters is also difficult, especially for longitudinal studies, due to increases in body size that can influence position of the tubing, as well as challenges with ensuring continued functionality (i.e., clogging, infection, etc.). Finally, several drugs that are abused by human adolescents are not readily self-administered by rodents, for example nicotine [134,135] and THC [136,137], the active ingredient in marijuana. As a result, virtually no studies exist testing the effects of marijuana SA in adolescent rats, and research on SA of other drugs during adolescence remains sparse. Literature exists on nicotine, as nicotine, like alcohol, will be self-administered by rats with training [138,139] , and adolescents may experience fewer aversive effects than adults following injections of nicotine [140,141] . Data are also available on the effects of adolescent SA of cocaine and heroin, and so we review results from that research below as well.
Nicotine Self-Administration in Adolescent Rats
Gender differences in nicotine SA exist. Adolescent males (PND 40-46) self-administered nearly triple the amount of nicotine of adult males, although intake tapered off after 2 weeks as the males approached adulthood (~PND 60), such that adolescent males did not self-administer greater amounts of nicotine as adults [117,144]. Chen and colleagues also reported that in Lewis rats, adolescent females acquired nicotine SA more rapidly than adults, and adolescent exposure caused females to self-administer significantly more nicotine as adults. Other studies in males agree that pre-exposed males do not self-administer more as adults [120], although contrary to the results reported by Levin and colleagues, these studies failed to find higher levels of SA for males during adolescence. Together these findings suggest that nicotine SA can be established in adolescent animals, and adolescents may be prone to self-administer greater amounts of nicotine than adults. Females appear to be more sensitive to the prolonged rewarding effects of nicotine, as they appear to be more likely to continue high levels of nicotine intake into adulthood. Some research suggests that hormonal differences could mediate the rewarding effects of nicotine [145,146], as well as possible gender differences in post-synaptic densities of nicotinic receptors [119,147-149], although future research needs to ascertain the role of each of these on reward and nicotine function.
Cocaine Self-Administration in Adolescent Rats
Conversely, Wong et al. recently reported that adolescent rats acquired cocaine SA more quickly and with lower doses than adults [153]. Adolescents also exhibited increased DA activity in the VTA of the brain, although this contrasts with what Frantz and colleagues reported for DA levels in the NAcc following cocaine exposure [153]. Anker and Carroll [154] also found that male adolescent Wistar rats self-administered more cocaine than adults, and they exhibited greater levels of cocaine-seeking behavior during extinction, suggesting that adolescents may be more vulnerable to relapse following addiction to cocaine. Another study by this group [122] reported that adolescents are more prone to SA cocaine than adults, and that female adolescents exhibit the greatest vulnerability for addiction as indexed by highest SA rates for cocaine. These authors suggested that failures to find significant differences in cocaine SA between adolescents and adults in previous studies was likely the result of testing doses which were too high (>0.4 mg/kg/infusion) or giving rats insufficient access time (< 2 hours per day). Future studies should continue to assess differences between adolescent and adult response to cocaine, to determine if adolescent SA may lead to increased vulnerability for addiction.
Heroin Self-Administration in Adolescent Rats
Reward Sensitivity during Adolescence
To assess the rewarding properties of stimuli, it is useful to determine the amount of effort an organism is willing to exert to obtain a reinforcer. Indeed, a hallmark of human addiction is the devotion of time, energy, and resources to obtain the addictive substance to the exclusion of other activities. It is assumed that compulsive behaviors are due to do a dysfunction in the brain reward system, such that alternative activities no longer carry reinforcing value. In animals, it is possible to assess reward value or at least motivation to obtain a substance by measuring “response cost” with operant schedules of reinforcement. For example, low fixed-ratio (FR) schedules like FR 1 or FR 2 have low response cost; the organism needs to exert little effort, merely one (FR 1) or two (FR 2) responses, to obtain access to a reinforcer. Increasing the FR requirement increases the cost accordingly, so that higher FR schedules can be used to determine what “price” animals are willing to pay in order to gain access to the reward. Another way to assess reward or motivation is to determine “breakpoint” on progressive ratio (PR) schedules of reinforcement. In PR schedules, increasingly higher numbers of responses are required to obtain a reinforcer, and the “breakpoint” is the point at which animals stop responding. Breakpoints offer an index of reward where higher breakpoints reflect higher reward value, as animals are willing to exert more effort to obtain the same reinforcer. PR schedules may be arranged in a number of ways. One simple method uses a fixed number of additive responses, for example PR 5 or PR 10, where response value must increase on each subsequent trial by N + PR to obtain additional reinforcers.
It has been challenging to assess FR or PR effects for alcohol in outbred strains of rats due to the trouble with establishing operant alcohol self-administration. Nevertheless, Rowland and colleagues [97] developed a unique PR design using access to a polyglucose gelatin matrix as the reinforcer. Adult male Sprague-Dawley rats were trained on an FR 1 schedule of reinforcement where lever presses resulted in access to 0.28 g of gelatin or gelatin plus 10% ETOH. The FR schedule was gradually increased to FR 30 over a period of three months, and then switched to a PR schedule. ETOH-infused gelatin was not more rewarding than gelatin alone, as determined by similar breakpoints to both reinforcers. However, intermittent forced exposure to an intoxicating dose of ETOH (3 g/kg, IP) later increased breakpoints on a PR 10 schedule from 28 to ~38 lever presses for ETOH-containing gelatin. These findings suggest that intermittent exposure to high doses of alcohol (perhaps similar to binge drinking in humans) during adulthood can increase the rewarding value of alcohol. In adolescents, Alaux-Cantin et al. [108] found that pre-exposure to 8 intoxicating doses of ETOH (3 g/kg, IP) increased breakpoints to gain access to 0.1 ml of 10% ETOH. However, these same rats did not show preference for alcohol in a CPP test following either 0.5 or 1.5 g/kg ETOH, making it difficult to conclude that adolescents found alcohol more rewarding. Future studies should continue to compare breakpoints and response cost between adolescents and adults self-administering alcohol.
A few studies have assessed reward valence of nicotine in adolescent versus adult rats using FR and PR schedules [120,144,165]. In contrast to findings from CPP studies, which generally report that adolescents demonstrate preference for places associated with nicotine (see [132] for review), adolescent rats seem to experience less reward from nicotine than adults when assessed with operant measures. For example, Shram and colleagues [165] found that adolescent rats self-administered significantly less IV nicotine than adults at doses of 0.015 and 0.03 mg/kg/infusion, and under a PR schedule of reinforcement, adolescents displayed breakpoints nearly half that of adults. Further, adolescents were less willing to respond for nicotine infusions when the response cost was increased slightly from FR 2 to FR 5 [165]. Sharp and colleagues also reported a decrease in responding for 0.03 mg/kg/infusion of IV nicotine when “price” was increased from FR 1 or FR 3 to FR 5 or FR 7 [120]. Levin et al. [144] found that when switched from a high-cost FR 8 to a less costly FR 1 schedule of reinforcement, adolescent rats continued to respond at a high rate, increasing nicotine intake nearly 8-fold. The authors inferred that adolescents experienced less reward from nicotine since they continued to self-infuse high doses [144], although there are alternative interpretations to this, including decreased aversive effects, or a lack of behavioral inhibition that motivated the high rates of response in these adolescents.
Only a few studies have assessed the rewarding properties of cocaine during adolescence with operant measures. Wong et al. [153] found that post-pubertal male adolescent Sprague-Dawley rats (PND 42) self-administered significantly more IV cocaine than either prepubertal (PND 35) or adult rats (PND 88) at doses ranging from 0.15- 0.60 mg/kg/infusion. Moreover, adolescents demonstrated increased motivation to obtain cocaine compared to adults, as they continued to respond for cocaine infusions even as price increased from FR 3 to FR 24. Insofar as motivation to bar press for cocaine infusions gauges reward, these results suggest that adolescents find cocaine more rewarding. In an effort to determine whether this increase in subjective reward was mediated by brain reward pathways, Wong and colleagues [153] hypothesized that the increased density and firing rate of DA-containing cells within the VTA (described above) motivates higher SA rates during adolescence. If true, then decreasing DA firing in adolescent rats should lower SA rates to adult levels, and increasing DA firing in adults should raise SA rates to that of adolescents. To decrease DA firing rates, Wong et al. gave IP injections of 0.2 mg/ kg of the D2 autoreceptor agonist quinpirole, a dose found to inhibit DA cell bodies without having appreciable effects on D2 receptors directly. As expected, decreasing DA firing rates with quinpirole decreased adolescent SA rates to adult levels, while increasing DA firing rates with the D2 antagonist eticlopride caused adult rats to increase their SA to match adolescents. Collectively, these results suggest that adolescents derive greater reward from cocaine, an effect which is mediated by increased levels of DA within the VTA.
In studies with heroin, Doherty and Frantz [164] found that adolescents self-administered more drug when cost was low (FR 1 to FR 5 schedules of reinforcement). Yet when price was increased on a PR schedule of reinforcement, adolescents did not exhibit differences in breakpoints from adults. These results suggested that adolescents were not willing to exert more effort to obtain the drug. On the basis of this limited data, it does not appear that adolescents experience greater reward from heroin and possibly other opiates, although future research must verify these results.
Based on these studies, it appears that adolescents may experience greater reward from DA agonists like cocaine that directly increase synaptic concentrations of DA. Dopamine agonists may enhance reward during adolescence due to their ability to stimulate the higher number of DA neurons within the striatal reward pathway. On the other hand, drugs with indirect actions on DA such as alcohol, nicotine and heroin do not appear to increase subjective feelings of reward, and in the case of nicotine, may actually decrease reward valence during adolescence. Attenuated reward following non-DA drugs may be the result of downstream reductions in synaptic DA or downregulation of D2 receptors, which have been found to occur following adolescent exposure to nicotine and alcohol [39,166,167]. However, this research is in its infancy and additional studies are necessary before firm conclusions may be drawn. Future studies should continue to test the hypothesis that directly enhancing DA can increase reward during adolescence. The rewarding effects of other DA agonists including amphetamine and methamphetamine, and perhaps non-abused DA agonists such as nomifensine, should be examined more carefully in adolescent populations. Studies should also directly compare the reward value of dopaminergic and non- DA drugs of abuse in adolescents, in an effort to determine whether stimulating DA during adolescence can increase subjective feelings of reward and possibly motivate addiction.
Conclusions and Future Directions
While several laboratories corroborate that adolescent rats are prone to voluntarily ingest greater amounts of alcohol, nicotine, cocaine, and heroin than adults [100,104,112,117,118,140,153,154,164,168,169] , the impact of adolescent pre-exposure on adult drug use has not been fully elucidated. Some studies of alcohol use report increased adult intake following adolescent exposure (both passive exposure and SA) [75,87,106-108,131,170] , but others do not [39,82,88,91,104,130] . Studies with nicotine generally report that adolescent SA increases adult intake in female rats [117,118,165], although at least 2 studies have not found an impact of adolescent SA in male rats [165,169]. Adolescent rats do not always prefer to selfadminister cocaine [150,152], and adolescent pre-exposure does not appear to increase motivation to take cocaine or heroin as an adult in rats [152,164]. These findings make it difficult to ascertain that drug exposure during adolescence modifies brain reward signaling to increase addiction potential.
Instead, it is well documented that adolescents are more prone to risk-taking and preference for novelty than adults, which is perhaps a biologically adaptive mechanism to promote greater diversity in mate selection [11,26]. Risk-taking, sensation-seeking, and preference for novelty motivate both humans and animals to seek out new experiences, which may be relevant to drug SA. Studies have shown that rats self-administering high doses of alcohol [91,104] and nicotine [117] during adolescence decreased their intake significantly by young adulthood. This pattern of alcohol and drug use mimics that of human teens, who often escalate drug intake during high school and college, but taper use in early adulthood (after age 23) [83,84,124]. In humans, decreased consumption in adulthood hasbeen attributed to competition between family and work obligations that leave less time to indulge in alcohol and drug use. However, evidence from rodent studies demonstrate that rats also decrease alcohol and nicotine intake in adulthood, independent of increased responsibility associated with human development. These findings suggest that there are distinct behavioral and/or neurobiological changes in adulthood that prompt decreased drug intake. Future research must strive to determine the exact underpinnings of this decline in substance abuse observed in adulthood, as understanding these mechanisms could lead to the development of treatments to temper substance abuse during adolescence.
Yet there remains a subpopulation of young adolescents who engage in extremely risky alcohol and drug use very early in life, and these teens appear at greatest risk for later abuse and addiction problems. Based on animal research, there does not appear to be a causal mechanism between exposing the brain to alcohol and drugs in early adolescence and later addiction. Instead, it appears that there are other genetic and environmental factors influencing both the tendency to experiment with drugs early in life and later addiction, such as a higher propensity for sensation-seeking and risk-taking, a lack of parental involvement, family history of addiction problems, etc. [83,133,171]. Future research in animals can help to elucidate these factors. For example, there is a high level of individual difference in the amount of alcohol intake among rats. Future studies should focus on comparing behavioral and neurobiological differences between adolescent “high-users” and “low users.” Pre-exposure assessment should also occur, in an effort to determine whether brain differences are present prior to the onset of SA, or if certain organisms appear more susceptible to permanent neurobiological changes following drug exposure that could motivate long-term addiction problems.
References
- Witt ED (2010) Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol 44: 119-124.
- Witt ED (1994) Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol 62: 168-177.
- Matthews DB (2010) Adolescence and alcohol: recent advances in understanding the impact of alcohol use during a critical developmental window. Alcohol 44: 1-2.
- Casey BJ , Rebecca MJ, Todd AH (2008) The adolescent brain. Annals of the New York Academy of Sciences 1124: 111-126.
- BJ Casey, Rebecca MJ, Leah HS (2011) Braking and Accelerating of theAdolescent Brain. J Res Adolesc 21: 21-33.
- Bava S , Tapert SF (2010) Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev 20: 398-413.
- Wahlstrom D, White T, Luciana M (2010) Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34: 631-648.
- Salamone JD , Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470-485.
- Söderpalm B, Ericson M (2013) Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci 13: 127-161.
- Ernst M, Romeo RD, Andersen SL (2009) Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav 93: 199-211.
- Crews FT, Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93: 237-247.
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, et al. (2006) Thedevelopment of cortical connections. Eur J Neurosci 23: 910-920.
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP (2010) Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn 72: 114-123.
- Spear L (2000) Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24: 115-123.
- Casey BJ, Jones RM (2010) Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry 49: 1189-1201.
- Crews FT, Boetigger AC (2009) Impulsivity, Frontal Lobes and Risk forAddiction. Pharmacol Biochem Behav 93: 237-247.
- Oades RD (1998) Frontal, temporal and lateralized brain function in children with attention-deficit hyperactivity disorder: a psychophysiological and neuropsychological viewpoint on development. Behav Brain Res 94: 83-95.
- Taylor E (1999) Developmental neuropsychopathology of attention deficit and impulsiveness. Dev Psychopathol 11: 607-628.
- Chambers RA, Jane RT, Marc NP (2003) Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160: 1041-1052.
- Geier CF, R Terwilliger, T Teslovich, K Velanova, B Luna (2010) Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex 20: 1613-1629.
- Goldman-Rakic PS (1996) The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci 351: 1445-1453.
- Fuster JM (2000) Executive frontal functions. Exp Brain Res 133: 66-70.
- Damasio AR (1995) On some functions of the human prefrontal cortex. Ann N Y Acad Sci 769: 241-251.
- Robbins TW (1996) Dissociating executive functions of the prefrontal cortex.Philos Trans R Soc Lond B Biol Sci 351: 1463-1470.
- Chudasama Y, Robbins TW (2006) Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73: 19-38.
- Crews F, He J, Hodge C (2007) Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86: 189-199.
- Eva HT, Andrew JF, Matthew DL, Adriana Galváne (2013) Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience 3: 45-52.
- Galván A, McGlennen KM (2013) Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. J Cogn Neurosci 25: 284-296.
- Somerville LH, Jones RM, Casey BJ (2010) A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn 72: 124-133.
- Bufill E, Agustí J, Blesa R (2011) Human neoteny revisited: The case of synaptic plasticity. Am J Hum Biol 23: 729-739.
- Selemon LD (2013) A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3: 1-9.
- Markham JA, Morris JR, Juraska JM (2007) Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 144: 961-968.
- Wise RA (1978) Catecholamine theories of reward: a critical review. Brain Res 152: 215-247.
- Kadzielawa K (1974) Dopamine receptor in the reward system of the rat. Arch Int Pharmacodyn Ther 209: 214-226.
- Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2: 695-703.
- 36 Cardinal RN (2006) Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw 19: 1277-1301.
- Hooks MS, Kalivas PW (1995) The role of mesoaccumbens--pallidal circuitry in novelty-induced behavioral activation. Neuroscience 64: 587-597.
- Pierce RC, Crawford CA, Nonneman AJ, Mattingly BA, Bardo MT (1990) Effect of forebrain dopamine depletion on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav 36: 321-325.
- Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108: 920-931.
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, et al. (2013) Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend [Epub ahead of print].
- Koob GF, Weiss F (1992) Neuropharmacology of cocaine and ethanol dependence. Recent Dev Alcohol 10: 201-233.
- Ritz MC, Kuhar MJ, George FR (1992) Molecular mechanisms associated with cocaine effects. Possible relationships with effects of ethanol. Recent Dev Alcohol 10: 273-302.
- O’Donnell P (2010) Adolescent maturation of cortical dopamine. Neurotox Res 18: 306-312.
- Andersen SL, Dumont NL, Teicher M (1997) Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol 356: 173-181.
- Andersen SL, LeBlanc CJ, Lyss PJ (2001) Maturational increases in c-fos expression in the ascending dopamine systems. Synapse 41: 345-350.
- Chen YI, Choi JK, Xu H, Ren J, Andersen SL, et al. (2010) Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev Neurosci 32: 125-138.
- Tarazi FI, Baldessarini RJ (2000) Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18: 29-37.
- Benes FM, Vincent SL, Molloy R, Khan Y (1996) Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse 23: 237-245.
- Benes FM, Taylor JB, Cunningham MC (2000) Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex 10: 1014-1027.
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, et al. (2013) Frontal Lobe γ-Aminobutyric Acid Levels During Adolescence: Associations with Impulsivity and Response Inhibition. Biol Psychiatry 74: 296-304.
- Anderson KJ, Mason KL, McGraw TS, Theophilopoulos DT, Sapper MS, et al. (1999) The ontogeny of glutamate receptors and D-apartate binding sites in the ovine CNS. Brain Res Dev Brain Res 118: 69-77.
- Bedi KS, Bhide PG (1988) Effects of environmental diversity on brain morphology. Early Hum Dev 17: 107-143.
- Lewis MH (2004) Environmental complexity and central nervous system development and function. Ment Retard Dev Disabil Res Rev 10: 91-95.
- van Praag H, Kempermann G, Gage FH (2000) Neural consequences of environmental enrichment. Nat Rev Neurosci 1: 191-198.
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, et al. (2010) Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ 17: 1092-1103.
- Gulley JM, Juraska JM (2013) The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience 249: 3-20.
- White AM, Swartzwelder HS (2004) Hippocampal function duringadolescence: a unique target of ethanol effects. Ann N Y Acad Sci 1021: 206-220.
- LM Squeglia, J Jacobus, SF Tapert (2009) The influence of substance use on adolescent brain development. Clin EEG Neurosci 40: 31-38.
- Guerri C, Pascual M (2010) Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44: 15-26.
- McBride WJ, Li TK (1998) Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12: 339-369.
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM, (2012) Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res 1466: 24-32.
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 25, 541-550.
- Coleman LG Jr, He J, Lee J, Styner M, Crews FT (2011) Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res 35: 671-688.
- Ehlers CL, Criado JR (2010) Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol 44: 27-37.
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS (2012) In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res 36: 279-285.
- Joohwi L, Cindy E, Fulton C, Marc N, Francois, et al. (2011) Automatic cortical thickness analysis on rodent brain. Proc Soc Photo Opt Instrum Eng 7962: 7962481-79624811.
- Elofson J, Gongvatana W, Carey KB (2013) Alcohol use and cerebral white matter compromise in adolescence. Addict Behav 38: 2295-2305.
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, et al. (2012) Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology 220: 529-539.
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, et al. (2000) Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry 157: 737-744.
- Spear LP, Brake SC (1983) Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16: 83-109.
- Odell WD, Swerdloff RS (1976) Etiologies of sexual maturation: a model system based on the sexually maturing rat. Recent Prog Horm Res 32: 245-288.
- Spear LP, Varlinskaya EI (2010) Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol 52: 236-243.
- Lester D, Nachman M, Le Magnen J (1970) Aversive conditioning by ethanol in the rat. Quarterly Journal of Studies on Alcohol 31, 578-586.
- van der Kooy D, O’Shaughnessy M, Mucha RF, Kalant H (1983) Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav 19: 441-445.
- Fidler TL, Bakner L, Cunningham CL (2004) Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav 77: 731-743.
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM (2011) Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res 225: 104-109.
- Morales M, Spear LP (2013) Differences in sensitivity to ethanol-induced conditioned taste aversions emerge after pre- or post-pubertal gonadectomy in male and female rats. Behav Brain Res 240: 69-75.
- Morales M, Varlinskaya EI, Spear LP (2012) Evidence for conditioned place preference to a moderate dose of ethanol in adult male Sprague-Dawley rats. Alcohol 46: 643-648.
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE (2010) Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology 35: 1453-1463.
- Grant KA, Samson HH (1985) Oral self administration of ethanol in free feeding rats. Alcohol 2: 317-321.
- Grant KA, Samson HH (1985) Induction and maintenance of ethanol selfadministration without food deprivation in the rat. Psychopharmacology 86: 475-479.
- Slawecki CJ, Betancourt M (2002) Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol 26: 23-30.
- McArdle P (2008) Use and misuse of drugs and alcohol in adolescence. BMJ 337: 46-50.
- Silveri MM (2012) Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry 20: 189-200.
- Roberts AJ, Heyser CJ, Koob GF (1999) Operant self-administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res 23: 1151-1157.
- Matthews DB, Overstreet DH, Rezvani AH, Devaud LL, Morrow AL (2001) Effects of sweetened ethanol solutions on ethanol self-administration and blood ethanol levels. Pharmacol Biochem Behav 68: 13-21.
- Nicholas WG, Chrisanthi AK, Heather NR (2012) Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PloS One 7: e31466.
- Broadwater M, Varlinskaya EI, Spear LP (2013) Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res 37: 1048-1055.
- Walker BM, Walker JL, Ehlers CL (2008) Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol 42: 83-89.
- McGregor IS, Saharov T, Hunt GE, Topple AN (1999) Beer consumption in rats: the influence of ethanol content, food deprivation, and cocaine. Alcohol 17: 47-56.
- Hargreaves GA, Monds L, Gunasekaran N, Dawson B, McGregor IS (2009) Intermittent access to beer promotes binge-like drinking in adolescent but not adult Wistar rats. Alcohol 43: 305-314.
- Lancaster F, Spiegel K, Zaman M (1987) Voluntary beer drinking in rats. Alcohol Drug Res 7: 393-403.
- Samson HH, Denning C, Chappelle AM (1996) The use of nonalcoholic beer as the vehicle for ethanol consumption in rats. Alcohol 13: 365-368.
- Cacace S, Plescia F, Sardo P, Cannizzaro C (2012) Alcohol preference, behavioural reactivity and cognitive functioning in female rats exposed to a three-bottle choice paradigm. Behav Brain Res 234: 11-19.
- Cacace S, Plescia F, La Barbera M, Cannizzaro C (2011) Evaluation of chronic alcohol self-administration by a 3-bottle choice paradigm in adult male rats. Effects on behavioural reactivity, spatial learning and reference memory. Behav Brain Res 219: 21-220.
- Peris J, Zharikova A, Li Z, Lingis M, MacNeill M, et al. (2006) Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav 85: 562-568.
- Li Z, Zharikova A, Vaughan CH, Bastian J, Zandy S, et al. (2010) Intermittent high-dose ethanol exposures increase motivation for operant ethanol selfadministration: possible neurochemical mechanism. Brain Res 1310: 142-153.
- Rowland NE, Nasrallah N, Robertson KL (2005) Accurate caloriccompensation in rats for electively consumed ethanol-beer or ethanolpolycose mixtures. Pharmacol Biochem Behav 80: 109-114.
- Orrù A, Fujani D, Cassina C, Conti M, Di Clemente A, et al. (2012) Operant, oral alcoholic beer self-administration by C57BL/6J mice: effect of BHF177, a positive allosteric modulator of GABA(B) receptors. Psychopharmacology 222: 685-700.
- Hargreaves GA, Wang EY, Lawrence AJ, McGregor IS (2011) Beer promotes high levels of alcohol intake in adolescent and adult alcohol-preferring rats. Alcohol 45: 485-498.
- Binakonsky J, Giga N, Ross C, Siegel M (2011) Jello shot consumption among older adolescents: a pilot study of a newly identified public health problem. Subst Use Misuse 46: 828-835.
- Spear LP, Varlinskaya EI (2005) Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol 17: 143-159.
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL (2012) Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav 102: 540-548.
- Vetter CS, Doremus-Fitzwater TL, Spear LP (2007) Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res 31: 1159-1168.
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, et al. (2011) Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav 100: 90-97.
- Criado JR, Ehlers CL (2013) Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacol Biochem Behav 103: 622-630.
- Blomeyer D, Friemel CM, Buchmann AF, Banaschewski T, Laucht M, et al. (2013) Impact of Pubertal Stage at First Drink on Adult Drinking Behavior. Alcohol Clin Exp Res [Epub ahead of print].
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, et al. (2013) Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67: 521-531.
- Roine RP, Gentry RT, Lim RT Jr, Baraona E, Lieber CS (1991) Effect of concentration of ingested ethanol on blood alcohol levels. Alcohol Clin Exp Res 15: 734-738.
- Council NNA (2004) NIAAA Council approves definition of binge drinking. NIAAA Newsletter: 3-5.
- Schulteis G, Archer C, Tapert SF, Frank LR (2008) Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol 42: 459-467.
- Doremus TL, Brunell SC, Rajendran P, Spear LP (2005) Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res 29:1796-1808.
- Brunell SC, Spear LP (2005) Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res 29: 1641-1653.
- Margaret B, Elena IV,Linda PS (2011) Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res 35: 1392-1403.
- Vetter-O’Hagen C, Varlinskaya E, Spear L (2009) Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol 44: 547-554.
- Tambour S, Brown LL, Crabbe JC (2008) Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res 32: 2100-2106.
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, et al. (2007) Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol 29: 458-465.
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS (2003) Adolescent-onset nicotine self-administration modeled in female rats Psychopharmacology (Berl) 169: 141-149.
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, et al. (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151: 392-405.
- Chen H, Matta SG, Sharp BM (2007) Acquisition of nicotine se/ fadministration in adolescent rats given prolonged access to the drug Neuropsychoparmacology 32: 700-709.
- Lynch WJ (2008) Acquisition and maintenance of cocaine selfadministrationin adolescent rats: effects of sex and gonadal hormones.Psychopharmacology (Berl) 197: 237-246.
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME (2011) Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology (Berl) 215: 785-799.
- Vetter-O’Hagen CS, Spear LP (2011) The effects of gonadectomy on ageand sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res 35: 2039-2049.
- Chartier KG, Hesselbrock MN, Hesselbrock VM (2010) Development and vulnerability factors in adolescent alcohol use. Child Adolesc Psychiatr Clin N Am 19: 493-504.
- Grant BF, Dawson DA (1998) Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 10: 163-173.
- Hingson RW, Heeren T, Winter MR (2006) Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med 160: 739-746.
- Meyers JL, Dick DM (2010) Genetic and environmental risk factors for adolescent-onset substance use disorders. Child Adolesc Psychiatr Clin N Am 19: 4650-477.
- Biondolillo KD, Pearce AR (2007) Availability influences initial and continued ingestion of nicotine by adolescent female rats. Neuropsychobiology 55: 73-80.
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM (2010) High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol 52: 424-440.
- Tolliver GA, Samson HH (1991) The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav 38: 575-580.
- Walker BM, Ehlers CL (2009) Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci 123: 926-935.
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM (2009) Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 206: 1-21.
- McCambridge J, McAlaney J, Rowe R (2011) Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLoS Med 8: e1000413.
- Belluzzi JD, Wang R, Leslie FM (2005) Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30: 705-712.
- Henningfield JE, Goldberg SR (1983) Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav 19: 989-992.
- van Ree JM, Slangen JL, de Wied D (1978) Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther 204: 547-557.
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G (2005) Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 81: 285-299.
- Cox BM, Goldstein A, Nelson WT (1984) Nicotine self-administration in rats. Br J Pharmacol 83: 49-55.
- Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99: 473-478.
- Shram MJ, Lê AD (2010) Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res 206: 240-244.
- Shram MJ, Funk D, Li Z, Lê AD (2006) Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 186: 201-208.
- Slotkin TA (2002) Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol 24: 369-384.
- Abreu-Villaça Y, Seidler FJ, Tate CA, Slotkin TA (2003) Nicotine is a neurotoxin in the adolescent brain: critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Res 979: 114-128.
- TLevin ED, Slade S, Wells C, Cauley M, Petro A, et al. (2011) Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res 225: 473-481.
- Lynch WJ (2009) Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav 94: 43-50.
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE (2009) Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 206: 303-312.
- Raval AP (2011) Nicotine addiction causes unique detrimental effects on women’s brains. J Addict Dis 30: 149-158.
- Pogun S, Yararbas G (2009) Sex differences in nicotine action. Handb Exp Pharmacol 192: 261-291.
- Perkins KA, Donny E, Caggiula AR (1999) Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1: 301-315.
- Frantz KJ, O’Dell LE, Parsons LH (2007) Behavioral andneurochemical responses to cocaine in periadolescent and adult rats.Neuropsychopharmacology 32: 625-637.
- Kerstetter KA, Kantak KM (2007) Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 194: 403-411.
- Li C, Frantz KJ (2009) Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 204: 725-733.
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M (2013) Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci 33: 4913-4922.
- Anker JJ, Carroll ME (2010) Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 208: 211-222.
- Compton WM, Volkow ND (2006) Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81:103-107.
- Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, et al. (2012) Opioid epidemic in the United States. Pain Physician 15: ES9-38.
- Hopfer CJ, Khuri E, Crowley TJ, Hooks S (2002) Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat 23: 231-237.
- Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, et al. (2012) Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy 23: 37-44.
- Lankenau SE, Schrager SM, Silva K, Kecojevic A, Bloom JJ, et al. (2012) Misuse of prescription and illicit drugs among high-risk young adults in Los Angeles and New York. J Public health Res 1: 22-30.
- Fibbi M, Silva K, Johnson K, Langer D, Lankenau SE (2012) Denial of prescription opioids among young adults with histories of opioid misuse.Pain Med 13: 1040-1048.
- Doherty J, Ogbomnwan Y, Williams B, Frantz K (2009) Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav 92: 164-172.
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, et al. (2009) Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. NeuroPsychopharmacology 34: 912-922.
- Doherty JM, Cooke BM, Frantz KJ (2013) A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. NeuroPsychopharmacology 38: 446-454.
- Doherty JM, Frantz KJ (2012) Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology (Berl) 219: 763-773.
- Shram MJ, Funk D, Li Z, Lê AD (2008) Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. NeuroPsychopharmacology 33: 739-748.
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA (2001) Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain res 892: 269-280.
- Maldonado-Devincci AM, Badanich KA, Kirstein CL (2010) Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol 44: 57-66.
- Perry JL, Anderson MM, Nelson SE, Carroll ME (2007) Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav 91: 126-133.
- Shram MJ, Li Z, Lê AD (2008) Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl) 197: 45-58.
- Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, et al. (2012) Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depend 125: 307-312.
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, et al. (2012) Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry 169: 39-46.
- Milivojevic V, Covault J (2013) Alcohol exposure during late adolescence increases drinking in adult Wistar rats, an effect that is not reduced by finasteride. Alcohol Alcohol 48: 28-38.