Journal of Andrology & Gynaecology
Erectile Dysfunction and Cardiovascular Disease: A Review
Charles N Walker1*, Stephanie M Meller2, Erik Stilp3, and Carlos Mena-Hurtado3
- 1Yale University School of Medicine, Department of Surgery, Section of Urology, USA
- 2Yale University School of Medicine, New Haven, CT, USA
- 3Yale University School of Medicine, Department of Internal Medicine, Section of Cardiovascular Medicine, USA
*Address for Correspondence: Charles Walker, MD, Yale University School of Medicine, Departmentof Urology, 789 Howard Avenue, FMP 323, New Haven, CT 06519, USA, E-mail: charles.walker@yale.edu
Citation: Walker CN, Meller SM, Stilp E, Mena-Hurtado C. Erectile Dysfunction and Cardiovascular Disease: A Review. J Androl Gynaecol. 2013;1(2): 10.
Copyright © 2013 Walker CN, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology |ISSN: 2332-3442 | Volume: 1, Issue: 2
Submission: 26 August 2013 | Accepted: 07 October 2013 | Published: 10 October 2013
Abstract
Erectile dysfunction (ED) is defined as the inability to achieve or maintain a penile erection for satisfactory sexual performance and it is estimated that >300 million men will suffer from this condition in 2025. ED is recognized as a harbinger of cardiovascular disease. Endothelial dysfunction and macrovascular atherosclerotic disease together represent the probable pathophysiological link between vasculogenic ED, coronary artery disease (CAD) and peripheral vascular disease (PAD), and research in these common areas in recent years has led to the emergence of a compelling body of evidence to support erectile dysfunction as the sentinel clinical event. The bidirectional relationship between low testosterone and components of metabolic syndrome (MetS) supports the conclusion that normal sex hormone production is an integral component for both metabolic and sexual health for the development of subsequent cardiac events and an improvement in overall health.Keywords: Erectile Dysfunction; Cardiovascular Disease; Endothelial Dysfunction, Risk
Introduction
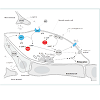
Despite its early description and documentation in 1948, erectile dysfunction (ED) has only been recognized as an organic failure of the normal neurovascular function of the penis within the past 2 to 3 decades [1]. Defined as the inability to achieve or maintain a penile erection for satisfactory sexual performance, ED affects >50% of men aged 40 to 70 years and 70% of men aged 70 years or older [2,3]. Moreover, the prevalence increases with age and is expected to further rise, potentially affecting over 300 million men worldwide by the year 2025 [4]. Organic, vasculogenic ED can result from aberrations in arterial and venous flow, endothelial and cavernosal smooth muscle function, and tunica albuginea compliance. The most common form of vascular ED results from penile arterial insufficiency, which will serve as the main focus for this review. Atherosclerotic occlusion or narrowing of the common iliac arteries, internal iliac arteries, and the internal pudendal arteries and their downstream branches may cause ED. Chronic arteriolar insufficiency leads to diminished neuronal and endothelial nitrous oxide (NO), and therefore causes impaired cavernosal smooth muscle relaxation resulting in ED.The Relationship between Erectile Dysfunction and Cardiovascular Disease
The Massachusetts Male Aging Study established the association between ED and CAD, demonstrating a 39% probability of complete ED in men with heart disease and subsequent studies have shown rates of ED in patients with CAD as high as 75% [2,8,9].Early impairment of endothelial dependent vasodilation has been shown in both vasculogenic ED and CAD. Without bioavailable NO, the vascular smooth muscle fails to relax and impedes vasodilation, necessary for erectile function. Kaiser et al. evaluated flow mediated vasodilation of the brachial artery in men with vasculogenic ED, as confirmed by penile Doppler, compared to age matched controls without ED. Men with ED had significantly impaired brachial artery vasodilation comparable to that seen in men with hyperlipidemia and early atherosclerotic disease. Treatment with PDE-5 inhibitors led to improvement in ED suggesting that the impairment in vasodilation involves the NO-cGMP system. Responsiveness to sublingual nitroglycerin was also reduced suggesting that the defect directly involves smooth muscle. That the impairment of vasodilation was shown to be both endothelium dependent and independent in this study is consistent with both a defect in eNOS bioavailability and intrinsic dysfunction of the smooth muscle. The authors concluded that in addition to the artery size hypothesis, a very plausible explanation for the manifestation of ED before other forms of vascular disease is the dependence of the penile vasculature on NO for vasodilation to a degree much greater than is seen in other vascular beds [38].
In patients with CAD and PAD, common cardiovascular risk factors create a pro-inflammatory, highly oxidative stressful state that stimulates free radical formation and atherogenesis leading to impaired NO release in such vascular beds. Similarly, obesity, diabetes, and MetS represent chronic inflammatory states and have been shown to damage the vascular endothelium and impair NO release in men with ED [39-41]. Men with obesity have been found to have impairment in multiple indices of endothelial function and significantly elevated levels of C-reactive protein [42]. In other studies recombinant C- reactive protein has been shown to downregulate endothelial NO and to promote endothelial apoptosis [43]. In a study of overweight patients with ED and without clinical evidence of DM, peripheral vascular disease, or CAD, circulating levels of endothelial progenitor cells (EPC) were reduced as compared to matched controls without ED, the extent of which correlated with severity of ED. Reduced levels of EPC, shown to be an independent predictor of cardiovascular disease, were also shown to independently predict ED in multivariate analysis [44]. Free radical formation, in addition to reducing bioavailability of NO, also potentiates atherosclerosis through direct damage to the endothelium. NO inhibits plateletaggregation and adhesion and smooth muscle proliferation. Thus reduced bioavailability of NO is associated with vasoconstriction, platelet adhesion, and smooth muscle cell proliferation, which further the atherosclerotic burden of the penile vasculature [45].
Further evidence for the role of endothelial dysfunction in ED comes from studies in diabetic men with ED and in animal models. A comparative study on corpus cavernosal and penile resistance arterial tissue from diabetic and non-diabetic men with ED demonstrated that the functional deficiency of NO found in men with ED is exacerbated in diabetes, which correlates with impaired endotheliumdependent relaxation in these patients [46]. Platelet aggregation and blood pressure response to L-arginine administration, surrogates for endothelial function, are believed to be mediated by NO, and have been shown to be lower in diabetic men with ED compared to diabetic patients without ED [47]. In addition, patients with ED have higher levels of asymmetric dimethylarginine, a known inhibitor of eNOS [48]. Insulin-like growth factor binding protein, which regulates the availability of insulin-like growth factor (IGF-1), is increased in hyperglycemic rats. Treatment with IGF-1 results in improvement in rat intracavernosal pressure and expression of endothelial NOsynthase [49-51] . Further evidence for the role of insulin resistance on endothelial NO production comes from the observation in obese rats that metformin administration induces eNos expression in penile tissue, via activated protein kinase [52].
Independent of NO regulation, there is evidence for the role of endothelin-1 in endothelial dysfunction in diabetes. Insulin stimulates production of endothelin-1, a potent vasoconstrictor, and NO in vascular endothelium. In non-diabetic individuals the vasodilatory effects of NO predominate; however, in insulin resistant states this does not occur. Accordingly, preservation of endothelin in the face of impaired nitric oxide production has been demonstrated in insulin resistant states [33].
Testosterone Deficiency, Metabolic Syndrome, and ED
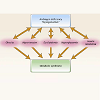
The relationship between testosterone deficiency and the MetS is significant and bidirectional [53]. Testosterone deficiency has been shown to be independently associated with the MetS as well as individual components of the syndrome. The two processes are tied by a number of shared pathophysiologic pathways including insulin resistance, hyperglycemia, dyslipidemia, visceral fat accumulation, inflammation, and endothelial dysfunction (Figure 3). Evidence for this comes from studies demonstrating that low levels of total testosterone in patients with ED have been associated with higher BMI, waist circumference, MetS, and insulin resistance, and treatment with testosterone can reduce central adiposity and insulin resistance [6,54,55]. In a prospective study of 1709 men, Kupelian et al. showed that low levels of total testosterone sex hormone binding globulin and symptomatic androgen deficiency were predictive of developing MetS with a RR of 1.41, 1.65, and 2.51 respectively [25]. The reciprocal nature of the relationship between the MetS and low testosterone is supported by a study of aging adults with low testosterone the results of which suggest a causative role for MetS in the development of hypogonadism [56]. Components of the MetS may directly promote hypogonadism through a number of mechanisms including decreased production of testosterone by Leydig cells, which is mediated by increased leptin levels in obesity. Visceral obesity and diabetes are both pro-inflammatory states and the production of a number of inflammatory cytokines have been shown to directly inhibit testosterone production, specifically TNF-α [57]. Central obesity leads to increased aromatase activity resulting in conversion of testosterone to increased levels of estradiol (E2), which suppresses LH release through hypothalamic negative feedback resulting in a functional state of hypogonadotropic hypogonadism [58].The role that testosterone deficiency plays in the evolution of both ED and CAD remains complex and controversial and while a full investigation of this subject is beyond the scope of this review, the topic is worthy of further discussion. It is generally well accepted that libido, frequency of sexual activity, and spontaneous (morning, nocturnal) erections are all testosterone dependent. Support for this comes from studies on hypogonadal men treated with testosterone replacement therapy [59]. Testosterone exerts its effects on erectile function in a number of ways. Centrally, testosterone acts directly on supraspinal centers of sexual function in the preoptic area and paraventricular nucleus of the hypothalamus, where it stimulates the production and release of erectogenic neurotransmitters, such a dopamine and oxytocin. Peripherally, testosterone is required for the normal function of sacral spinal neurons involved in reflexogenic erections and it regulates parasympathetic nerves in corpus cavernosum. Animal studies have shown that testosterone regulates the production of both endothelial and neuronal nitric oxide synthases and androgen suppression has been shown to cause significant reduction in both NOS activity and that of cGMP in the corpus cavernosum [60,61].
Testosterone is also directly required for maintenance of the integrity of both the endothelium and the smooth muscle. Low testosterone is associated with impaired flow mediated vasodilation, a surrogate for endothelial dysfunction [62]. In animal models of castration, atrophy and fibrosis of corporal cavernosal tissue is found to be in part due to the reduction of trabecular smooth muscle content and an increase in components of extracellular matrix [61]. Androgen withdrawal also leads to apoptosis of corporal cavernosal smooth muscle [63]. Animal models and human studies have shown that PDE5 expression is also regulated by testosterone [64,65]. In hypogonadal men with ED that is refractory to PDE5 inhibitors, supplementation with testosterone have been shown to improve erectile function and increase penile arterial flow as indicated by increased cavernosal peak systolic velocities [66,67]. Further evidence for the role of testosterone in the maintenance of normal erectile physiology comes from animal studies showing relaxation of corporal smooth muscle after treatment with testosterone [63].
Clinically, low levels of total and bioavailable testosterone have been associated with erectile dysfunction [68]. Low testosterone is associated with both hypoactive desire and erectile dysfunction in a dose dependent fashion such that impairment of sexual function is seen when testosterone levels drop below the lower limit of the normal adult range [69]. Corona and colleagues found in their study of 1647 men that low testosterone levels were associated with the severity of ED and the magnitude of penile blood flow [70].
However, reports of the prevalence of hypogonadism in men with ED are variable in the literature and depend in part on the threshold of testosterone used, whether total testosterone (TT), free testosterone (FT) or bioavailable testosterone (BT) are used to determine prevalence as well as whether or not determinations of testosterone are repeated. Assessment of total testosterone in men with ED reveals a prevalence of hypogonadism between 5-15% with rates of 20-40% being found when determinations of free and bioavailable testosterone are used [71-73]. Threshold levels of testosterone for impairment of sexual function are highly variable between individuals. Consequently there is no standardized lower limit of testosterone, however several definitions have been proposed. The lower limit of normal for total testosterone accepted by the FDA is 300 ng/dL while the recommendation from a 2007 position paper by the Endocrine Society is a testosterone level of < 200 ng/ml (6.9 nM) [74]. For practical purposes the determination of hypogonadism depends on both the determination of testosterone below a threshold level and the presence of symptoms, including reduced sexual drive, impotence, infertility, fatigue, depression or irritability, decreased bone density, loss of lean muscle mass, anemia, and increased body fat.
Both the production and bioavailability of testosterone are reduced with aging at a rate of 0.5%-2% per year [75-78].
ED, Low Testosterone, and Cardiovascular Mortality
Given the extent of the association between low testosterone, MetS, endothelial dysfunction, ED, and CVD, it is not surprising that both low testosterone and ED are independent predictors of increased cardiovascular mortality [70]. The relationship between ED and mortality has been demonstrated in a number of studies. Vlachopoulos et al. in their meta-analysis of 14 cohort studies found a RR of 1.19 and 1.23 for cardiovascular and all cause mortality respectively in men with ED [79]. In their meta-analysis of 7 cohort studies of Guo et al. also found that men with ED were at increased risk for all cause mortality with a RR of 1.23 though only two studies reported information on all cause mortality [80]. In a case control study of 291 diabetic men with angiographically confirmed silent CAD, ED significantly predicted an increased risk for major cardiac event defined as CAD Death, sudden death, nonfatal myocardial infarction, death due to congestive heart failure, unstable angina, need for repeat revascularization, stroke or TIA, and symptomatic peripheral artery disease with a hazard ratio of 2.1 [31].The Role of Risk Modification
A Selective phosphodiesterase type 5 (PDE-5) inhibitors (sildenafil, vardenafil, and tadalafil) enhance NO-mediated relaxation of the corpus cavernosum via increased intracavernosal cGMP levels, resulting in erection initiation and maintenance [9]. Given their ease of use and excellent safety profile, PDE-5 inhibitors are recommended as first line drug therapy; however, these drugs demonstrate lower response rates in older men. The reasons for this age discrepancy include an age-associated decrease in endogenous NO production, which is further diminished by other comorbidities [87]. Of the 30-40% of patients who do not initially respond, counseling and daily low-dose administration may be helpful, and in men with low testosterone, testosterone replacement therapy has been shown to improve response to PDE-5 inhibitor therapy by increasing the bioavailable NO in the cavernous smooth muscle tissue [88].Guidelines for Management of the Patient with ED and No Evidence of Cardiovascular Disease
The accumulation of evidence supporting ED as a predictor of CAD indicates that ED and hypogonadism should serve as potential warning signs of future cardiovascular disease [32]. As proposed by Miner et al., a “window of curability” may exist in which treatment may stop the progression of cardiovascular disease and potentially ED, especially in men < 60 years old, where the cardiovascular risk portended by ED appears to be especially high [53,112].Conclusion
The link between vasculogenic ED and CVD is well supported by the literature and very likely indicates that the disease processes are distinct manifestations of the same atherosclerotic process. ED may be the first overt manifestation of potentially serious cardiovascular disease. Because vasculogenic ED typically presents earlier than CVD, clinicians should recognize the condition as an early warning sign of future cardiovascular pathology particularly in diabetic men.References
- Kinsey AC, Pomeroy WB, Martin CE (1948) Sexual behavior in the human male.
- Johannes CB AA, Feldman HA, Derby CA, Kleinman KP, McKinlay JB, et al. (2000) Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the massachusetts male aging study. J Urol 163: 460-463.
- Chew KK, Finn J, Stuckey B, Gibson N, Sanfilippo F, et al. (2010) Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: Findings from a linked-data study. J Sex Med 7: 192-202.
- Aytac IA, Araujo AB, Johannes CB, Kleinman KP, McKinlay JB (2000) Socioeconomic factors and incidence of erectile dysfunction: Findings of the longitudinal massachussetts male aging study. Soc Sci Med 51: 771-778.
- Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, et al. (2005) The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol 96: 19M-23M.
- Guay A, Jacobson J (2007) The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med 4:1046-1055.
- Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, et al. (2006) Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab 290: E234-242.
- Kloner RA, Mullin SH, Shook T, Matthews R, Mayeda G, et al. (2003) Erectiledysfunction in the cardiac patient: How common and should we treat? J Urol 170: S46-50.
- Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, et al. (2003) Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol 44: 360-364.
- Kaiser FE, Viosca SP, Morley JE, Mooradian AD, Davis SS, et al. (1988) Impotence and aging: Clinical and hormonal factors. J Am Geriatr Soc 36: 511-519.
- Rogers JH, Karimi H, Kao J, Link D, Javidan J, et al. (2010) Internal pudendal artery stenoses and erectile dysfunction: Correlation with angiographic coronary artery disease. Catheter Cardiovasc Interv 76: 882-887.
- Montorsi P, Ravagnani PM, Galli S, Rotatori F, Veglia1F, et al. (2006) Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: The cobra trial. Eur Heart J 27: 2632-2639.
- Virag R, Bouilly P, Frydman D (1985) Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet 1: 181-184.
- Montorsi P, Ravagnani PM, Galli S, Briganti A, Salonia A, et al. (2005) Association between erectile dysfunction and coronary artery disease: A case report study. J Sex Med 2: 575-582.
- Ma RC, So WY, Yang X, Yu LW, Kong AP, et al. (2008) Erectile dysfunction predicts coronary heart disease in type 2 diabetes. J Am Coll Cardiol 51: 2045-2050.
- Thompson IM TC, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, et al. (2005) Erectile dysfunction and subsequent cardiovascular disease. JAMA 294: 2996-3002.
- Greenstein A CJ, Chen J, Miller H, Matzkin H, Villa Y, et al. (1997) Does severity of ischemic coronary disease correlate with erectile function? Int J Impot Res 9: 123-126.
- Solomon H, Man JW, Wierzbicki AS, Jackson G (2003) Relation of erectile dysfunction to angiographic coronary artery disease. Am J Cardiol 91: 230- 231.
- Foroutan SK, Rajabi M (2007) Erectile dysfunction in men with angiographically documented coronary artery disease. Urol J 4: 28-32.
- Riedner CE, Rhoden EL, Fuchs SC, Wainstein MV, Gonçalves SC, et al. (2011) Erectile dysfunction and coronary artery disease: An association of higher risk in younger men. J Sex Med 8: 1445-1453.
- Kawanishi Y, Lee KS, Kimura K, Koizumi T, Nakatsuji H, et al. (2001) Screening of ischemic heart disease with cavernous artery blood flow in erectile dysfunctional patients. Int J Impot Res 13:100-103.
- El-Sakka AI, Morsy AM, Fagih BI, Nassar AH (2004) Coronary artery risk factors in patients with erectile dysfunction. J Urol 172: 251-254.
- Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ, et al. (2006) Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 166: 207-212.
- Shabsigh R, Fishman IJ, Schum C, Dunn JK (1991) Cigarette smoking and other vascular risk factors in vasculogenic impotence. Urology 38: 227-231.
- Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB (2006) Erectile dysfunction as a predictor of the metabolic syndrome in aging men: Results from the massachusetts male aging study. J Urol 176: 222-226.
- Heidler S, Temml C, Broessner C, Mock K, Rauchenwald M, et al. (2007) Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol 177: 651-654.
- Esposito K, Giugliano F, Martedi E, Feola G, Marfella R, et al. (2005) High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes care 28: 1201-1203.
- Wei M MC, Davis DR, Hornung CA, Nankin HR, Blair SN, et al. (1994) Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol 140: 930-937.
- Kobat MA , Fırdolas F, Balin M, Celik A, Bentli R, et al. (2012) Circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are associated with erectile dysfunction in patients without known coronary artery disease. J Sex Med.
- Garcia-Malpartida K, Marmol R, Jover A, Gomez-Martinez MJ, Sola- Izquierdo E, et al. (2011) Relationship between erectile dysfunction and silent myocardial ischemia in type 2 diabetic patients with no known macrovascular complications. J Sex Med 8: 2606-2616.
- Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, et al. (2008) Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: A potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol 51: 2040-2044.
- Corona G, Mannucci E, Forti G, Maggi M (2009) Hypogonadism, ed, metabolic syndrome and obesity: A pathological link supporting cardiovascular diseases. Int J Androl 32: 587-598.
- Stehouwer CD, Henry RM, Ferreira I (2008) Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 51: 527-539.
- Yamanaka M, Shirai M, Shiina H, Tanaka Y, Tsujimura A, et al. (2003) Diabetes induced erectile dysfunction and apoptosis in penile crura are recovered by insulin treatment in rats. J Urol 170: 291-297.
- Rey-Valzacchi GJ, Costanzo PR, Finger LA, Layus AO, Gueglio GM, et al. (2012) Addition of metformin to sildenafil treatment for erectile dysfunction in eugonadal nondiabetic men with insulin resistance. A prospective, randomized, double-blind pilot study. J Androl 33: 608-614.
- Lue TF (2003) Erectile dysfunction. N Engl J Med 342:1802-1813.
- Simonsen U, Garcia-Sacristan A, Prieto D (2002) Penile arteries and erection. J Vasc Res 39:283-303.
- Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL,et al. (2004) Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. Journal of the American College of Cardiology. 43:179-184.
- Martinez-Jabaloyas JM (2013) Prevalence of co-morbidities in patients with erectile dysfunction. Actas Urol Esp 37: 33-39.
- Bansal TC GA, Jacobson J, Woods BO, Nesto RW (2005) Incidence of metabolic syndrome and insulin resistance in a population with organic erectile dysfunction. J Sex Med 2:96-103.
- Potenza MA, Montagnani M (2008) Abnormal insulin signaling: Early detection of silent coronary artery disease-erectile dysfunction? Curr Pharm Des 14: 3737-3748.
- Giugliano F, Esposito K, Di Palo C, Ciotola M, Giugliano G (2004) Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Invest 27: 665- 669.
- Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, et al. (2002) A selffulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106: 913-919.
- Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, et al. (2009) Circulating cd34+ kdr+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med 6: 107-114.
- Creager MA, Luscher TF, Cosentino F, Beckman JA (2003) Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part i. Circulation 108:1527-1532.
- Angulo J, Gonzalez-Corrochano R, Cuevas P, Fernandez A, La Fuente JM, et al. (2010) Diabetes exacerbates the functional deficiency of no/cgmp pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med 7: 758-768.
- De Angelis L, Marfella MA, Siniscalchi M, Marino L, Nappo F, et al. (2001) Erectile and endothelial dysfunction in type ii diabetes: A possible link. Diabetologia 44:1155-1160.
- Elesber AA, Solomon H, Lennon RJ, Mathew V, Prasad A, et al. (2006) Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis. Eur Heart J 27: 824-831.
- Soh J, Katsuyama M, Ushijima S, Mizutani Y, Kawauchi A, et al. (2007) Localization of increased insulin-like growth factor binding protein-3 in diabetic rat penis: Implications for erectile dysfunction. Urology 70:1019- 1023.
- Pu XY HL, Wang HP, Luo YX, Wang XH (2007) Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J Androl 9: 83-91.
- Pu XY, Wen AM, Zheng XG, Liu JM, Zhou XX, et al. (2012) [insulin-like growth factor-1 gene therapy improves the levels of mrna and protein of endothelial nitric oxide synthase in aging related erectile dysfunction in rats]. Zhonghua yi xue za zhi 92:128-130.
- Kim YW, Park SY, Kim JY, Huh JY, Jeon WS, et al. (2007) Metformin restores the penile expression of nitric oxide synthase in high-fat-fed obese rats. J Androl 28: 555-560.
- Miner MM (2012) Men’s health in primary care: An emerging paradigm of sexual function and cardiometabolic risk. Urol Clin North Am 39:1-23.
- Knoblovits P, Costanzo PR, Valzacchi GJ, Gueglio G, Layus AO, et al. (2010) Erectile dysfunction, obesity, insulin resistance, and their relationship with testosterone levels in eugonadal patients in an andrology clinic setting. J Androl 31: 263-270.
- Stanworth RD, Jones TH (2008) Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging 3: 25-44.
- Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, et al. (2011) Testosterone and metabolic syndrome: A meta-analysis study. J Sex Med 8: 272-283.
- Hong CY, Park JH, Ahn RS, Im SY, Choi HS, et al. (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 24: 2593-2604.
- Vermeulen A, Kaufman JM, Deslypere JP, Thomas G (1993) Attenuated luteinizing hormone (lh) pulse amplitude but normal lh pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 76: 1140-1146.
- Buvat J, Shabsigh R, Guay A, Gooren L, Torres L, Mueleman E. Hormones, metabolism, aging and men’s health. In: Porst H, Buvat J, Medicine TSCotISfS, eds.(2006) Standard practice in sexual medicine. Massachusettes, USA: Blackwell Publishing 225-286.
- Park KH, Kim SW, Kim KD, Paick JS (1999) Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int 1999 83: 327-333.
- Traish A, Kim N (2005) The physiological role of androgens in penile erection: Regulation of corpus cavernosum structure and function. J Sex Med 2:759- 770.
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, et al. (2007) Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 30:1029-1034.
- Alcorn JF, Toepfer JR, Leipheimer RE (1999) The effects of castration on relaxation of rat corpus cavernosum smooth muscle in vitro. J Urol 161: 686-689.
- Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, et al. (2005) Testosterone regulates pde5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol 47: 409-416.
- Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, et al. (2004) Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology 145: 2253-2263.
- Aversa A, Isidori AM, Spera G, Lenzi A, Fabbri A (2003) Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. Clin Endocrinol 58:632-638.
- Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H (2004) Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 172: 658-663.
- Kratzik CW, Schatzl G, Lunglmayr G, Rucklinger E, Huber J (2005) The impact of age, body mass index and testosterone on erectile dysfunction. J Urol 174: 240-243.
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, et al. (2001) Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281: E1172-1181.
- Corona G, Rastrelli G, Monami M, Guay A, Buvat J, et al. (2011) Hypogonadism as a risk factor for cardiovascular mortality in men: A metaanalytic study. Eur J Endocrinol 165: 687-701.
- Buvat J, Lemaire A (1997) Endocrine screening in 1,022 men with erectile dysfunction: Clinical significance and cost-effective strategy. J urol 158:1764- 1767.
- Bodie J, Lewis J, Schow D, Monga M (2003) Laboratory evaluations of erectile dysfunction: An evidence based approach. J urol 169: 2262-2264.
- Earle CM, Stuckey BG (2003) Biochemical screening in the assessment of erectile dysfunction: What tests decide future therapy? Urology 62: 727-731.
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H (2007) Position statement: Utility, limitations, and pitfalls in measuring testosterone: An endocrine society position statement. J Clin Endocrinol Metab 2007 92: 405-413.
- Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, et al. (1997) Longitudinal changes in testosterone, luteinizing hormone, and folliclestimulating hormone in healthy older men. Metabolism 46: 410-413.
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C (2006) Prevalence of hypogonadism in males aged at least 45 years: The him study. Int J Clin Pract 60: 762-769.
- Snyder PJ (2001) Effects of age on testicular function and consequences of testosterone treatment. J Clin Endocrinol Metab 86: 2369-2372.
- Vermeulen A (2001) Androgen replacement therapy in the aging male--a critical evaluation. J Clin Endocrinol Metab 86:2380-2390.
- Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI (2013) Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: A systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes 6: 99-109.
- Guo W, Liao C, Zou Y, Li F, Li T, et al. (2010) Erectile dysfunction and risk of clinical cardiovascular events: A meta-analysis of seven cohort studies. J Sex Med 7: 2805-2816.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR (2006) Low serum testosterone and mortality in male veterans. Arch Intern Med 166: 1660-1665.
- Laughlin GA, Barrett-Connor E, Bergstrom J (2008 ) Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68-75.
- Haring R, Volzke H, Steveling A, Krebs A, Felix SB, et al. (2010) Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J 31:1494-1501.
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, et al. (2005) Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin Endocrinol 63: 280- 293.
- Boyanov MA, Boneva Z, Christov VG (2003)Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6:1-7.
- Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS (2001) Intramuscular testosterone esters and plasma lipids in hypogonadal men: A meta-analysis. Am J Med 111:261-269.
- Albersen M, Orabi H, Lue TF (2012) Evaluation and treatment of erectile dysfunction in the aging male: A mini-review. Gerontology 58: 3-14.
- Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H (2004) Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 172: 658-663.
- Fink HA, Mac Donald R, Rutks IR, Nelson DB, Wilt TJ (2002) Sildenafil for male erectile dysfunction: A systematic review and meta-analysis. Arch Intern Med 162:1349-1360.
- Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, et al. (2005)Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol 47: 214-220.
- Herrmann HC, Levine LA, Macaluso J, Jr., Walsh M, Bradbury D, et al. (2006) Can atorvastatin improve the response to sildenafil in men with erectile dysfunction not initially responsive to sildenafil? Hypothesis and pilot trial results. J Sex Med 3: 303-308.
- Juenemann KP, Muth S, Rohr G, Siegsmund M, Alken P (1990) Does lipid metabolism influence the pathogenesis of vascular impotence? Int J Impot Res 2: 33.
- Manning M, Schmidt P, Juenemann KP, Alken P ( 1996) The role of blood lipids in erectile failure. Int J Impot Res 8: D179.
- Ghofrani HA, Pepke-Zaba J, Barbera JA, Channick R, Keogh AM,et al. (2004) Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J Am Coll Cardiol 43: 68S-72S.
- Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC (2006) Vardenafil: A novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial k(atp) channels in rabbits. J Mol Cell Cardiol 40: 405-411.
- Oliver JJ, Melville VP, Webb DJ (2006) Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension 48: 622-627.
- Katz SD, Balidemaj K, Homma S, Wu H, Wang J, et al. (2000) Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol 36: 845- 851.
- Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M,et al. (2002) The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 40: 1232-1240.
- Salloum F, Yin C, Xi L, Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Cir Res 92: 595-597.
- Gupta BP MM, Clifton MM, Prokop L, Nehra A, et al. (2011) The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: A systematic review and meta-analysis. Arch Intern Med 171: 1797-1803.
- Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, et al. (2004) Effect of lifestyle changes on erectile dysfunction in obese men: A randomized controlled trial. JAMA 291: 2978-2984.
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, et al. (2000) Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? Urology 56: 302-306.
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, et al. (2003) Sexual function in men older than 50 years of age: Results from the health professionals follow-up study. Ann Intern Med 139:161-168.
- Nicolosi A, Glasser DB, Moreira ED, Villa M (2003) Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: A population study. Int J Impot Res 15: 253-257.
- Niebauer J, Cooke JP (1996) Cardiovascular effects of exercise: Role of endothelial shear stress. J Am Coll Cardiol 28:1652-1660.
- Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, et al. (2000) Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454-460.
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. (2006) Diet and lifestyle recommendations revision 2006: A scientific statement from the american heart association nutrition committee. Circulation 114: 82-96.
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008) Adherence to mediterranean diet and health status: Meta-analysis. BMJ 337: a1344.
- Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, et al. (2009) Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 119: 1093-1100.
- Esposito K, Ciotola M, Giugliano F, De Sio M, Giugliano G, et al. (2006) Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res 18: 405-410.
- Giugliano F, Maiorino MI, Bellastella G, Autorino R, De Sio M,et al. (2010) Adherence to mediterranean diet and erectile dysfunction in men with type 2 diabetes. J Sex Med 7: 1911-1917.
- Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, et al. (2009) A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc 84: 108-113.
- Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, et al. (2012) The princeton iii consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc 87: 766-778.
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, et al. (2010) 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 122: 2748-2764.
- Araujo AB, Hall SA, Ganz P, Chiu GR, Rosen RC, et al. (2010) Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the framingham risk score? J Am Coll Cardiol 55: 350-356.
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, et al. (2002) Acc/aha 2002 guideline update for exercise testing: Summary article. A report of the american college of cardiology/american heart association task force on practice guidelines (committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol 40:1531-1540.