Advances in Diabetes & Endocrinology
Download PDF
Case Report
*Address for Correspondence: Majid H Alabbood, Department of Medicine and Health Sciences, MacquarieUniversity, Australia, Tel: +61422411090; E-mail: dr_majid79@yahoo.com
Citation: Alabbood MH, Ling M, Ho KW. Triple Phase Response of Transient Hyponatremia or Secondary Adrenal Insufficiency? A Case Report. Adv Diabetes Endocrinol 2016; 1(1): 3.
Copyright © 2016 Alabbood MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Advances in Diabetes & Endocrinology | Volume: 1, Issue: 1
Submission: 26 August, 2016 | Accepted: 21 September, 2016| Published: 25 September, 2016
Triple Phase Response of Transient Hyponatremia or Secondary Adrenal Insufficiency? A Case Report
Majid H Alabbood*, Min Ling and Kenneth W Ho
- Department of Medicine and Health Sciences, Macquarie University, Australia
*Address for Correspondence: Majid H Alabbood, Department of Medicine and Health Sciences, MacquarieUniversity, Australia, Tel: +61422411090; E-mail: dr_majid79@yahoo.com
Citation: Alabbood MH, Ling M, Ho KW. Triple Phase Response of Transient Hyponatremia or Secondary Adrenal Insufficiency? A Case Report. Adv Diabetes Endocrinol 2016; 1(1): 3.
Copyright © 2016 Alabbood MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Advances in Diabetes & Endocrinology | Volume: 1, Issue: 1
Submission: 26 August, 2016 | Accepted: 21 September, 2016| Published: 25 September, 2016
Abstract
Hyponatremiais common in neurosurgical patients especially after pituitary surgery. It is the most common electrolyte abnormalityencountered in general hospital admissions. Diagnosis of the underlying cause can be challenging. It has serious clinical sequelaeif not diagnosed promptly, and can be fatal. Hyponatremia can result from severe hypothyroidism and secondary adrenal insufficiency. In this report, we describe a case of fluctuating sodium level in a middle aged male resembling triple phase response of transient hyponatremia. However, we suggest another diagnosis of hyponatremia due to secondary adrenal insufficiency followed by unmasking of underlying CDI by glucocorticoid replacement after resection of a large nonfunctioning pituitary macroadenoma.Keywords
Hyponatremia; Secondary adrenal insufficiency; Pituitary glandAbbreviations
ADH: Antidiuretic Hormone; CDI: Cranial Diabetes Insipidus; CRH: Corticotropin Releasing Hormone; CSWS: Cerebral SaltWasting Syndrome; FSH: Follicular Stimulating Hormone; FT4: Free T4; GH: Growth Hormone; H.C: Hydrocortisone; I.V: Intravenous;MRI: Magnetic Resonance Imaging; PEG: Polyethylene Glycol; PROP1: Prophet of Pit 1; SIADH: Syndrome of Inappropriate Secretion of Antidiuretic Hormone; UOP: Urine Output TG: Triglyceride; TSH: Thyroid Stimulating HormoneIntroduction
Hyponatremia, defined as serum sodium level less than 135 mmol/L [1-4], is common in neurosurgical patients especially after pituitary surgery. It has a reported incidence of 10-20% [3,5,6]. It is the most common electrolyte abnormality affecting general medical admissions with an estimated incidence of 2 to 30% [2,7,8]. Diagnosis of the underlying cause can be challenging. It has serious clinical sequelae if not diagnosed promptly, and can lead to fatal outcomes [3]. Endocrine disorders are uncommon causes of hyponatremia [7], and include severe hypothyroidism and secondary adrenal insufficiency [2-4,7]. Association of secondary adrenal insufficiency and hyponatremia was first described by Bethune and Nelson [9]. Hyponatremia was documented in 80% of patients with secondary adrenal insufficiency in one study that involved 31 elderly patients [1]. In this report, we present a case of fluctuating sodium level in a middle aged male resembling triple phase response of transient hyponatremia. However, we suggest another diagnosis of hyponatremia secondary to secondary adrenal insufficiency followed by unmasking of underlying CDI by glucocorticoid replacement.Case Report
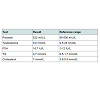
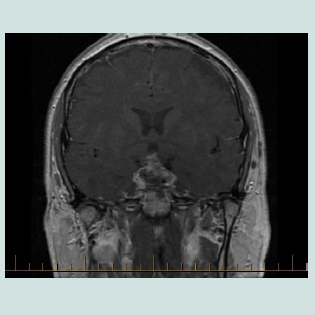
A 42-year-old male presented with bi-temporal hemianopia associated with a large non-functioning pituitary macroadenoma. Pre-operative pituitary hormonal assessment is shown in (Table 1). Post PEG recovery index of prolactin was 79%. Other laboratory tests are shown in Table 1. He underwent trans-sphenoidal resection. Intravenous hydrocortisone was commenced peri-operatively. Hissurgery was complicated by hematoma (Figure 1). He underwent evacuation of haematoma. Second MRI shown in Figure 2. Histopathology confirmed the diagnosis of pituitary adenoma expressing FSH. He initially developed Central Diabetes Insipidus (CDI) with urine output more than 300 ml/hour, hypernatraemia (147 mmol/L), serum hyper-osmolarity (307 mOsm/kg) and urine hypo-osmolality of 144 mOsm/kg. He was managed with intermittent 1 µg I.V desmopress in injections (7 times over 4 days). Serum sodium fell to 129 mmol/L with desmopressin administrations. His fluid intake was then restricted to 1.5 L/day. Over the next few days, serum sodium improved to 132 mmol/L. As his pre-operative cortisol was normal, hydrocortisone was ceased seven days post-operatively as per protocol. A day later, the patient complained of fatigue and dizziness. On examination, he was euvolemic, but with postural hypotension. Serum sodium was 132 mmol/L and serum osmolality was 280 mOsm/kg. Serum lipids were moderately elevated and serum protein was normal (Table 1).Discussion
The authors presented a case of hyponatremia in a young adult shortly after pituitary surgery. The serum sodium showed a triphasic pattern of hypernatremia followed by hyponatremia and finally hypernatremia. We are going to discuss the approach of hyponatremia first and then how to differentiate triple phase response of transient hyponatremia from SIADH like syndrome due to secondary adrenal insufficiency and unmasking of CDI by steroid replacement.In approaching hyponatremia, pseudohyponatremia (due to hypertriglyceridemia or hyperparaproteinemia) should be excluded first [2]. Although the index case showed elevated level of lipids as shown in Table 1, pseudohyponatremia was excluded for 3 reasons: first, there was clear serum hypotonicity. Second, the TG was not extremely elevated (less than 9 mmole/L). Third, the osmolar gap (the difference between measured and calculated serum osmolarity) was less than 14 mOsm/kg [2]. Upon clarification, the patient admitted a history of familial hyperlipidemia and he deliberately stopped hypolipidemic agent shortly prior to surgery.
Then we excluded primary polydipsia and reset osmostat syndrome, which is characterised by downward resetting of serum osmolality level to stimulate ADH secretion [2], by the presenceof impaired water excretion reflected by urine osmolality ≥ 100 mOsmol/kg which indicates complete and appropriate suppression of ADH [2]. Effective arterial blood volume depletion states wereexcluded by the urine sodium > 20 mmol/L [2]. Ultimately, our provisional diagnosis was limited to SIADH, CSWS, hypothyroidism and hypoadrenalism. CSWS was excluded by the lack of sign and symptoms of hypovolemia such as postural change in pulse rate, laboratory evidence of haemoconcentration, urine osmolality ofmore than 450 mOsm/kg and urine sodium concentration lower than 20 mmol/L. Furthermore, the low TSH and early morning serum cortisol confirmed the diagnosis of hypopituitarism which was the culprit of hyponatremia in this case. Also, normal potassium level supports the diagnosis of secondary rather than primary adrenal insufficiency. As expected, hyponatremia was dramatically improved after steroid replacement [3]. Interestingly, the glucocorticoid replacement corrected hyponatremia in the index case before thyroxine replacement was initiated which is similar to another case report [10]. This indicates that the hyponatremia was mainly due to adrenal insufficiency rather than hypothyroidism. Hyponatremia can be the only presenting feature of patients with secondary adrenal insufficiency and requires high index of suspicion. Hyponatremia was present in 80% of patients with secondary adrenal insufficiency in a study [1]. Several case reports support this conclusion [1,5,11,12]. Two case reports described hyponatremia secondary to Sheehan’s syndrome [13,14] despite the fact that the latter has classically been associated with CDI. Another case report described hyponatremia in primary empty sella syndrome [10]. Pekic et al. described a case ofhyponatremia in familial hypopituitarism due to PROP1 mutation [8]. However, the cases in these reports were mainly elderly [1,5,11,12,15] in contrasts to the index case which is a middle aged patient. similarity Jahangiri et al. study found that age is not a risk factor to develop postoperative hyponatremia [16]. Preoperativehypopituitarism is the single most predictive factor of postoperative hyponatremia [16]. This contrasts the index case whom preoperative pituitary assessment was normal.
Triple phase response which is characterized by hypernatremia due to CDI followed by a transient hyponatremia as result of the rapid release of pre stored ADH in the adenohypophysis due to surgical trauma to the stalk and finally hypernatremia secondary to permanent CDI [3,12]. Our patient’s pituitary stalk seemed relatively intact, as seen in the postoperative MRI scan (Figure 21,3]. Secondly, cortisol deficiency stimulates the up-regulation of aquaporin-2 on which ADH exerts its action at the collecting tubules [7]. Thirdly, hypotension which results from cortisol deficiency stimulates the production of ADH via baroreceptors [1,3,8]. Fourthly, cortisol deficiency increases water permeability at the collecting tubules [2].Fifthly, cortisol has an important role in the maintenance of the normal inhibition of vasopressin in response to hypotonicity andcortisol is a physiological tonic inhibitor of ADH [7,14]. Therefore, steroid replacement can unmask CDI and this is what occurred in the index case. The patient developed DI as a result of hypophysectomy which was treated effectively by desmopressin. Then when he developed secondary hypoadrenalism, the CDI state changed to a SIADH like syndrome which was corrected by steroid replacement. The later resulted in unmasking of a pre-existed and permanent CDI. This scenario is highly similar to triphasic CDI and it is hard to differentiate between the two conditions. However, the prompt response to steroid replacement and the lack of evidence of stalk trauma during surgery can help to differentiate.
In summary, hyponatremia may be the sole presenting feature of secondary adrenal insufficiency. Hypothyroidism should be excluded as well. Prompt replacement with corticosteroids followedby thyroxine is essential and lifesaving. Unmasking of permanent CDI can lead to rapid and over correction of hyponatremia, and this should be differentiated from “triple phase response” of transient hyponatremia.
References
- Asano T, Aoki A, Sasaki M, Ikoma A, Kakei M, et al. (2012) Hyponatremia is the valuable manifestation for initiating diagnosis of hypopituitarism in elderly. Endocr J 59: 1015-1020.
- Milionis HJ, Liamis GL, Elisaf MS (2002) The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ 166: 1056-1062.
- Cuesta M, Hannon MJ, Thompson CJ (2016) Diagnosis and treatment of hyponatraemia in neurosurgical patients. Endocrinol Nutr 63: 230-238.
- Mansell P, Scott VL, Logan RF, Reckless JP (1993) Secondary adrenocortical insufficiency. BMJ 307: 253-254.
- Akhtar S, Cheesman E, Jude EB (2013) SIADH and partial hypopituitarism in a patient with intravascular large B-cell lymphoma: a rare cause of a common presentation. BMJ Case Rep 2013: bcr2012007147.
- Oh JY, Shin JI (2015) Syndrome of inappropriate antidiuretic hormone secretion and cerebral/renal salt wasting syndrome: similarities and differences. Front Pediatr 2: 146.
- Liamis G, Milionis HJ, Elisaf M (2011) Endocrine disorders: causes of hyponatremia not to neglect. Ann Med 43: 179-187.
- Pekic S, Doknic M, Miljic D, Saveanu A, Reynaud R, et al. (2011) Case seminar: a young female with acute hyponatremia and a sellar mass. Endocrine 40: 325-331.
- Bethune JE, Nelson DH (1965) Hyponatremia in hypopituitarism. N Engl J Med 272: 771-776.
- Okuno S, Inaba M, Nishizawa Y, Miki T, Inoue Y, et al. (1987) A case of hyponatremia in panhypopituitarism caused by the primary empty sella syndrome. Endocrinol Jpn 34: 299-307.
- YDiederich S, Franzen NF, Bahr V, Oelkers W (2003) Severe hyponatremia due to hypopituitarism with adrenal insufficiency: report on 28 cases. Eur J Endocrinol 148: 609-617.
- Olchovsky D, Ezra D, Vered I, Hadani M, Shimon I (2005) Symptomatic hyponatremia as a presenting sign of hypothalamic-pituitary disease: a syndrome of inappropriate secretion of antidiuretic hormone (SIADH)-like glucocorticosteroid responsive condition. J Endocrinol Invest 28: 151-156.
- Bamoulid J, Courivaud C, Kazory A, Bonneville JF, Ducloux D (2009) The Case a female with hyponatremia. Kidney Int 76: 351-352.
- Kageyama Y, Hirose S, Terashi K, Nakayama S, Komatsuzaki O, et al. (1988) A case of postpartum hypopituitarism (Sheehan's syndrome) associated with severe hyponatremia and congestive heart failure. Jpn J Med 27: 337-341.
- Rodríguez-García J, Fraile G, Martínez V (1990) Inappropriate secretion of vasopressin, hypopituitarism and corticosteroid therapy. Postgrad Med J 66: 981-982.
- Jahangiri A, Wagner J, Tran MT, Miller LM, Tom MW, et al. (2013) Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations: Clinical article. J Neurosurg 119: 1478-1483.