Journal of Toxins
Download PDF
Research Article
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata 700009, India, Tel: 91-33-23508386; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Gomes A, Sarkar A, Ghosh S, Saha K, Saha PP et al. Edible Fresh Water Snail Viviparous Bengalensis Purified Flesh Protein VB-P4 Induced Toxicities and its Protection by Heat Treatment. J Toxins. 2015;2(2): 3
Copyright © 2015 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 2, Issue: 2
Submission: 03 October, 2015 | Accepted: 19 December, 2015 | Published: 23 December, 2015
In vivo rat cutaneous haemorrhage: Minimum haemorrhagic dose (MHD) of VB-P4 was found to be 10 μg. HT-VB-P4 (10 μg/ i.d/20 gm mice/24 hrs) did not produce any haemorrhagic spot as compared with saline treated control rats (Figure 3).
In vivo rat gastric haemorrhage/lesion: VB-P4 (40 μg/per oral/100 gm rat) produced gastric haemorrhagic and lesion spots, gastric necrosis in pylorus ligated male albino rats. HT-VB-P4 (40 μg/per oral/100 gm rat) did not produced gastric haemorrhagic and lesion spots, gastric necrosis in pylorus ligated male albino rats as compared with saline treated pylorus ligated male albino rats (Figure 4). Histological studies with VB-P4 (40 μg/per oral/100 gm rat/4 hrs) treated rat stomach showed gastric lesion spots, gastric epithelial breakage and necrosis as compared with saline treated pylorus ligated control rat stomach (Figure 5).
In vitro toxicity studies with VB-P4 & HT-VB-P4
Effect on isolated rat phrenic-nerve diaphragm preparation: VB-P4 and HT-VB-P4 (24 μg/6 ml bath volume) did not significantly altered the electrical induced twitch response on the isolated rat phrenic-nerve diaphragm preparation observed up to 60±5 min.
Edible Fresh Water Snail Viviparous Bengalensis Purified Flesh Protein VB-P4 Induced Toxicities and its Protection by Heat Treatment
Antony Gomes*1, Amrita Sarkar2, Sourav Ghosh1, Kalyani Saha1, Partha Pratim Saha3, TanmoyBhowmik 1 and Aparna Gomes 4
- 1Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata 700009, India
- 2Department of Translational Medicine, Suite 543, 1020 Locust Street, Thomas Jefferson University, Philadelphia, PA 19107, USA
- 3Department of Zoology, JK College, Purulia, West Bengal, India
- 4CSIR-Indian Institute of Chemical Biology, Kolkata 700032, India
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata 700009, India, Tel: 91-33-23508386; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Gomes A, Sarkar A, Ghosh S, Saha K, Saha PP et al. Edible Fresh Water Snail Viviparous Bengalensis Purified Flesh Protein VB-P4 Induced Toxicities and its Protection by Heat Treatment. J Toxins. 2015;2(2): 3
Copyright © 2015 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 2, Issue: 2
Submission: 03 October, 2015 | Accepted: 19 December, 2015 | Published: 23 December, 2015
Abstract
Nutritional and therapeutic values of the edible gastropod (snail) are well known throughout the world. Earlier from this laboratory, a protein VB-P4 was identified from the edible snail (Vivaparous bengalensis) flesh extract having anti-osteoporosis and antiosteoarthritis activity on albino Wistar rats. The present study was done on the in vivo/in vitro toxicities of VB-P4 and heat treated VBP4 (HT-VB-P4) on experimental animals. The in vivo toxicities of VB-P4/HT-VB-P4 was done in male albino Swiss mice treated for 15 days and assessed through haematological (total count of RBC/WBC, Hb%), biochemical markers (SGOT, SGPT, LDH, CK-MB, urea, creatinine) and histological studies of organs (liver and kidney). The in vitro toxicities of VB-P4/HT-VB-P4 was done on isolated guinea-pig heart/auricle (for cardiotoxicity), isolated rat phrenic nerve diaphragm preparation (for neurotoxicity), haemorrhagic activity (in vivo cutaneous and gastric haemorrhage), haemolytic activity on RBC (mice, rat, guinea-pig and goat) and rat peritoneal mast cell degranulation activity. It was observed that 15 days sub-chronic treatment in male albino mice with VB-P4/HT-VB-P4 did not significantly changed the total count of RBC, WBC and haemoglobin. However, 15 days sub-chronic treatment in male albino mice with VB-P4 significantly increased the liver markers (SGOT, SGPT), myotoxicity markers (LDH, CK-MB) but not the renal markers (Urea, Creatinine). HT-VB-P4 did not significantly change the liver markers (SGOT, SGPT), myotoxicity markers (LDH, CK-MB) and the renal markers (Urea, Creatinine). Histological studies with VB-P4 showed significant changes in liver tissue (fatty infiltration, necrotic lesions, dilated central vein) as compared with the control group of mice. Histological studies with VB-P4 did not show any significant changes in kidney tissue. In vitro cardiotoxicity was shown by VB-P4 on isolated guinea-pig heart but not on isolated guines-pig auricle. VB-P4 significantly decreased the heart rate and amplitude of contraction leading to irreversible blockade of contraction. HT-VB-P4 did not showed cardiotoxicity on the above preparations. VB-P4/HT-VB-P4 did not show neurotoxicity on isoalated rat phrenic-nerve diaphragm preparation. VB-P4 induced cutaneous haemorrhagic spots, gastric lesions in male albino rats through in vivo studies, which was not found with HT-VB-P4 treatment. Histological studies of stomach treated with VB-P4 confirmed the presence of blood, gastric lesions and necrotic spots, which was absent in HT-VB-P4 treatment. In in vitro studies, VBP4 produced haemolysis of RBC (mice, rat, guinea-pig and goat) and degranulated rat peritoneal mast cells, which was absent in case of HT-VB-P4 treatment. The data from this study confirmed that intake of raw snail flesh protein may induce toxicity especially in liver, stomach and RBC. Boiling/cooking before consumption may overcome the toxicities and safe for human consumption.Keywords
Viviparous bengalensis; Edible snail; Snail protein; in vivo toxicity; in vitro toxicityIntroduction
Viviparous bengalensis (VB), a fresh water edible snail, is a staple food for the socio-economically backward people of rural India as a cheap protein substitute source. In many countries (Philippines, Vietnam, Laos, Cambodia, China, Japan, northeast Indian states), people eat snail flesh in raw form as delicacy. In Indonesia, they are fried as satay, a dish known as sate kakul. In many countries, people use to boil snail in hot water before consumption. In India, snail cooked in mustard oil, used as an ointment over different types of sore and corn. In the north Bihar state of India, the flesh of Bellamia (Viviparous) bengalensis had been used as a traditional medicine against arthritis [1]. Snail flesh contained different types of protein, peptide, lipoproteins, oligosaccharides, enzymes of biological importance [2]. Gauri et al. purified and characterized a novel antibacterial peptide from Bellamia bengalensis which was found to be active against ampicillin and chloramphenicol resistance Staphylococcus epidermitis [3]. Sarkar et al. reported that Indian edible snail (Viviparous bengalensis) flesh extract possess antiosteoporosis and anti-osteoarthritis activity in experimental rats [4]. Recently, Sarkar et al. isolated a protein (VB-P4) from the snail (Viviparous bengalensis) flesh extract which was found to possess anti-osteoporosis and anti-osteoarthritis activity in albino rats [5,6]. VB-P4 had 562 amino acids, glycoprotein in nature and acted through pro/anti oxidants, serum cytokines and through T cell modulation. The present study was an effort to explore the toxic (both in vivo and in vitro) effect of fresh water edible snail flesh protein VB-P4 on experimental animals.Materials and Methods
Materials usedAll the materials and chemicals used were of analytical grade, unless otherwise mentioned. The following chemicals were used in the present study: Acetic acid, Anaesthetic ether, disodium hydrogen phosphate, DPX, Eosin, Folin phenol reagent, Haematoxylene, NaCl, sodium dihydrogen phosphate, Toluidine blue, Tri sodium isocitrate (SRL, India), Bovine serum albumin (Sigma-Aldrich, USA), CK-MB Kit, Creatinine Kit, LDH Kit, SGOT Kit, SGPT Kit, Urea Kit (Labkit, India), Drabkins reagent (Cogent, India), Ethanol (Bengal Chemicals, India), Paraffin (Merck, Germany).
Experimental animals
Swiss albino male mice (20±2 g), male albino Wistar rats (150±10 g) and male guinea pig (200±20 g) were procured from enlisted supplier of Calcutta University and housed in standard polypropylene cages at controlled environment (24 °C±2 °C, 12 hour light-dark cycle, relative humidity 60%±5%). The animals were provided with dry pellet diet, green vegetables, gram and water ad libitum. The experiments were conducted according to the departmental animal ethics committee for the purpose of control and supervision of experiments on animals (animal ethical reference no. PHY/CU/ IAEC/20/2008). All animal experiments were approved by the animal ethics committee, Department of Physiology, University of Calcutta and were in accordance with the guideline of the committee for the purpose of control and supervision of experiments on animal (CPCSEA), Government of India. For in vitro RBC hemolysis, goat blood was collected from slaughter’s house.
Collection of Viviparous bengalensis and purification of VBP4
Viviparous bengalensis live sample collection and purification was done as described earlier [4]. The snails (Viviparous bengalensis) were identified by the Zoological Survey of India. Snail flesh was dissected out and a 10% of the flesh homogenate was prepared in PBS (0.01 M, pH 7.2) using a glass homogenizer fitted with a Teflon piston (Remi Motors Ltd; India, model no. RQ-127A). The homogenate was centrifuged (8000 rpm x 30 min at 4 °C) and the supernatant was termed as Viviparous bengalensis extract (VBE) expressed in terms of protein content. Crude VBE was subjected to ion exchange chromatography (IEC) using DEAE cellulose column with phosphate buffer. Fractions were collected using graded NaCl molarity and all the fraction proteins were estimated after Lowry et al. [7]. Peak 4 (VBP4) was further analyzed through HPLC using C18 column (4.6×250 mm), methanol:water solvent system (70:30) with a flow rate 1 ml/ min and monitored at 280 nm.
Heat treatment of VB-P4
VB-P4 was kept at 80±2 °C for 20±2 min, cooled and centrifuged at 3000 rpm for 15 min. The supernatant was collected for protein estimation after Lowry et al. [7]. It was provisionally designated as HT-VB-P4 (heat treated Viviparous bengalensis protein 4).
In vivo toxicity studiesGrouping of animals and treatment: Male Swiss albino mice (20±2 g, n=6) were taken and divided into 3 groups- Gr.1: Control, Gr.2: VB-P4 treated (40 μg/20 gm /i.p/15 days) and Gr.3: HT-VB-P4 treated (40 μg/20 gm /i.p/15 days). On 16th day, blood was collected from hepatic portal vein (for haematological studies) and serum was separated for biochemical studies. Tissues (liver, kidney) were collected for histological studies.
Hematological and biochemical parameters: For hematological studies, blood was collected in heparinised vial and total count of WBC, RBC and hemoglobin percentage was done after Mukherjee et al. [8]. Serum was prepared from blood and SGOT, SGPT, Urea, Creatinine, LDH, CK-MB were analyzed with biochemical kit (Labkit, India) using UV spectrophotometer (Analab, model no UV-180).
Histological studies: Liver, kidney and stomach tissues were collected, fixed in 10% buffered formalin for 24 hrs. The tissues were then dehydrated in graded (50-100%) ethanol followed by clearing in Xylene. Paraffin (56-58 °C) embedding was done at 58±1 °C for 4 hrs, followed by paraffin block preparation. Paraffin section (5 μ) was cut using a rotary microtome (Weswox Optic, India). Paraffin sections were deparaffinised with xylene, stained with hematoxylineosin, followed by mounting in DPX with a cover slip. Histological characteristics of the tissues were observed with a bright field microscope (Motic, Germany) and photographs were captured with Motic software (Motic Images Plus 2.0 software).
In vivo rat cutaneous haemorrhage: This was performed according to the method of Kondo et al. [9]. VB-P4 (10 μg/i.d/20 gm mice/24 hrs) and HT-VB-P4 (10 μg/i.d/20 gm mice/24 hrs) was injected on the shaved dorsal flank on the Swiss albino male mice (n=4). Same amount of phosphate buffer (pH 7.4) was injected incontrol animal. The mice were sacrificed after 24 hour, skin was opened and observed for hemorrhagic lesion. Diameter of haemorrhagic spot was measured with a caliper and photograph was captured with a digital camera (Panasonic Lumix). Minimum haemorrhagic dose (MHD) was the dose of VB-P4 that produced a haemorrhagic spot of 10 mm diameter.
In vivo rat gastric haemorrhage/lesion: Male albino Wistar rats (150±10 g, n=4) were deprived of food for 72 hour (water was provided ad libitum) before experiment. The animals were lightly anaesthetized with anesthetic ether. A small slit was made in the abdomen and stomach pylorus ligation was done after Shay et al. [10]. The animals were orally fed with VB-P4/ HT-VB-P4 (40 μg/per oral/100 g rat) and after 4 hr, all the rats were sacrificed and the hemorrhagic spot, gastric lesion was photographed with a digital camera (Panasonic Lumix). Stomach tissue samples were fixed in formal-buffer for histopathological studies following the method as stated earlier.
In vitro toxicity studies
Effect on isolated guinea pig heart and auricle: Isolated guinea pig heart was prepared after Langendorff [11] and perfused with oxygenated (95% O2 + 5% CO2) Tyrode’s solution (NaCl 137 mM, KCl 2.7 mM, CaCl2·2H2O 2.6 mM, MgCl2·6H2O 1.27 mM, NaHCO3 11.9 mM, NaH2PO4 0.4 mM and glucose 5.5 mM) containing double dextrose (2 g l-1), temperature was maintained at 37±1 °C. The preparation was treated with VB-P4/HT-VB-P4 (40 μg/bolus) and contraction was recorded on a rotating smoked drum using a heart lever.Isolated guinea pig auricle was prepared after Burn and suspended in oxygenated Tyrode’s solution (29 ±1 °C) in a 4 ml glass bath [12]. The preparation was treated with VB-P4 and HT-VB-P4 (10 μg/4 ml bath volume) and spontaneous contraction was recorded on a smoked drum by a lightly sprung lever.
Effect on isolated nerve-muscle preparation: Isolated rat phrenic nerve diaphragm (RPND) was prepared after Bulbring and suspended in a 6 ml glass bath containing oxygenated (95% O2 + 5% CO2) Tyrode’s solution at 29±1 °C [13]. The preparations were stimulated with a square wave electronic stimulator of 8 V, 0.5 ms duration, 0.2 Hz (Grass, USA, Model No. SD9). RPND preparations were treated with VB-P4 and HT-VB-P4 (20 μg/5 ml bath volume) and contraction was recorded with Brodie’s lever on a smoked kymograph paper.
In vitro RBC haemolysis: Hemolytic activity of VB-P4/ HTVB-P4 was done according to Rothschild [14]. Blood (mice, rat, guinea-pig and goat) was collected in 3.8% citrate buffer, repeatedly washed with 0.9% saline and a 2% suspension of RBC (in 0.9% saline) was prepared. 1 ml RBC suspension was treated with VB-P4/ HT-VB-P4 (10-50 μg/ml RBC suspension), incubated at 37 °C for 30 min. Volume was made with chilled 0.9% saline and centrifuged at 3000 rpm for 15 min. The extent of hemolysis was estimated in the supernatant fluid (against saline treated control) at optical density of 540 nm. Distilled water was used as standard (100% lysis). Haemolytic dose 50 (HD50) was calculated graphically with the mice RBC haemolysis % vs. doses of VB-P4.
In vitro rat peritoneal mast cell degranulationPeritoneal membrane was collected in 0.9% saline from a freshly killed male albino Wistar rat. The Peritoneal membrane was cut in small pieces and placed in different watch glasses in 0.9% saline. Group 1: 0.5 ml 0.9% saline (control), Group 2: 0.5 ml 0.9% saline and VB-P4 (10-80 μg/ml) and Group 3: 0.5 ml 0.9% saline and HT-VB-P4 (10-80 μg/ml). The watch glasses were incubated at 37 °C for 30 min. After incubation, the peritoneum from each group was placed on clear glass slide in perfectly stretched condition and was fixed over flame. The slide was then treated with ethanol:chloroform:acetic acid (6:3:1) mixture for 15 min. After downgrading treatment with ethanol:water, the slides were stained with 0.1% aqueous toluidine blue. The excess stain was washed with distilled water and upgrading treatment with ethanol:water up to 100% ethanol. The slides were washed twice with xylene and mounted in DPX. The mast cell count was done under the light microscope and expressed in terms of % degranulation against control preparation [15]. Mast cell degralulation dose 50 (MCD50) of VB-P4 was calculated against saline treated control preparation.
Statistical analysis
Data were expressed as mean±SEM (n=6). Statistical analysis was done using one way analysis of variance (ANOVA). P< 0.05 was considered to be statistically significant.
Results
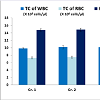
In vivo sub-chronic toxicity studies with VB-P4 & HTVB-P4VB-P4 & HT-VB-P4 (40 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice did not showed significant changes in blood total WBC, RBC and haemoglobin as compared with the control animals (Figure 1). VB-P4 (40 & 80 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice showed significant changes in serum GOT, GPT, LDH, CK-MB level but not in serum urea, creatinine level as compared with control group of mice. However, HT-VB-P4 (40 & 80 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice did not showed significant changes in serum GOT, GPT, LDH, CK-MB, urea, creatinine level as compared with control group of mice (Table 1).
Histological studies with VB-P4 (40 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice showed significant changes in liver tissue (fatty infiltration, necrotic lesions, dilated central vein) as compared with the control group of mice. HT-VB-P4 (40 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice did not showed significant changes in liver tissue (Figure 2). Histological studies with VB-P4 and HT-VB-P4 (40 μg/20 gm/ip x 15 days) treatment in male albino Swiss mice did not showed significant changes in kidney tissue as compared with the control group of mice (Figure 2).
Figure 2: Effect of VB-P4 and HT-VB-P4 on liver and kidney histologya) Liver tissue of control group 1 animal, b) Liver tissue of VB-P4 treated group 2 animal, c) Liver tissue of HT-VB-P4 treated group 3 animal, d) Kidney tissue of control group 1 animal, e) Kidney tissue of VB-P4 treated group 2 animal, f) Kidney tissue of HT-VB-P4 treated group 3 animal. (Hematoxyleneeosin stain, Magnification 150X).
In vivo rat gastric haemorrhage/lesion: VB-P4 (40 μg/per oral/100 gm rat) produced gastric haemorrhagic and lesion spots, gastric necrosis in pylorus ligated male albino rats. HT-VB-P4 (40 μg/per oral/100 gm rat) did not produced gastric haemorrhagic and lesion spots, gastric necrosis in pylorus ligated male albino rats as compared with saline treated pylorus ligated male albino rats (Figure 4). Histological studies with VB-P4 (40 μg/per oral/100 gm rat/4 hrs) treated rat stomach showed gastric lesion spots, gastric epithelial breakage and necrosis as compared with saline treated pylorus ligated control rat stomach (Figure 5).
Effect on isolated guinea-pig heart and isolated guinea-pig auricle: VB-P4 (40 μg/bolus) treatment on isolated guinea-pig heart significantly reduced the rate and amplitude of contraction (90±5%) leading to irreversible blockade within 8±2 min of observation (Figure 6A). However, HT-VB-P4 (40 μg/bolus) did not changed the rate and amplitude of contraction observed up to 60±5 min (Figure 6B).VB-P4 (10 μg/4 ml bath volume) treatment on isolated guineapig auricle significantly reduced (50±3%) the rate and amplitude of contraction within 8±2 min of observation (Figure 6C). Normal contraction regained within 38±5 min of observation. However, HT-VB-P4 (10 μg/4 ml bath volume) did not changed the rate and amplitude of contraction observed up to 75±8 min (Figure 6D).
Effect on isolated rat phrenic-nerve diaphragm preparation: VB-P4 and HT-VB-P4 (24 μg/6 ml bath volume) did not significantly altered the electrical induced twitch response on the isolated rat phrenic-nerve diaphragm preparation observed up to 60±5 min.
In vitro RBC haemolysis: VB-P4 (10-50 μg/ml RBC suspension) produced dose dependent haemolysis (15-90 %) of RBC of mice, rat, guinea-pig, goat. HD50 of VB-P4 on mice RBC was found to be 24±2 μg. HT-VB-P4 (10-50 μg/ml RBC suspension) did not produced haemolysis of RBC of mice, rat, guinea-pig, goat as compared with saline treated control RBC.
In vitro rat peritoneal mast cell degranulation: VB-P4 (10-80 μg/ml) produced dose dependent degranulation (11-80%) of rat peritoneal mast cells. MCD50 of VB-P4 was found to be 45±5 μg. HTVB-P4 (10-80 μg/ml) did not degranulated rat peritoneal mast cells as compared with saline treated control mast cells.
Discussion
Proteins are energy yielding, body building materials that are available from natural resources of the plant and animal kingdom. Many edible animal proteins (snail, mussel, crab, etc.) have toxicity on humans. Fresh water snail (Viviparous bengalensis) consumption is a source of low cost protein in under developed countries like India, Bangladesh, Vietnam, etc. In many countries, it is consumed in raw form. The question remains, whether it is safe to consume in raw form? The present study was explored with this idea that how far the snail flesh protein is safe for consumption in the raw form? This study confirmed that the snail flesh protein VB-P4 show toxicities in rodent system. Sub-chronic toxicities of VB-P4 did not show any toxicity of the blood profile (total count of RBC, WBC and haemoglobin) of the rats. VB-P4 showed liver toxicity through increased liver markers (SGOT, SGPT) in serum and myotoxicity through increased level of myotoxicity markers LDH, CK-MB. SGOT (serum glutamic oxaloacetic transaminase) or Aspartate transaminase (AST) is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that catalyzes the reversible transfer of an α-amino group between aspartate and glutamate [16] and is an important enzyme in amino acid metabolism. Aspartate transaminase catalyzes the interconversion of aspartate and α-ketoglutarate to oxaloacetate and glutamate. This enzyme mainly found in liver and down regulation of this enzyme show liver toxicity or liver problem. Lactate dehydrogenase, a cytochrome-C dependent enzyme that catalyzes the conversion of pyruvate to lactate [17]. Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+. It converts pyruvate, the final product of glycolysis, to lactate when oxygen is absent or in short supply and it performs the reverse reaction during the Cori cycle in the liver. In a typical patient with acute myocardial infarction serum LDH level exceeds the normal range within 24 to 48 hours, reaches a peak elevation of two- to tenfold in 3 to 6 days [18]. The CK-MB is a cardiac marker to diagnose acute myocardial infarction [19]. In cardiac tissue, CK-MB act as energy buffer by converting creatine to phosphocreatine by using inorganic phosphate (Pi) from ATP, creatine phosphate reserves the energy and helps to produce ATP during requirement. Following the onset of an acute myocardial infarction, intracellular CK-MB release into serum from the area of infarction and elevated the serum CK-MB level [20].VB-P4 also showed myotoxicity on isolated guinea-pig heart, where it produced cardiac blockade. It is likely that the cardiac blockade was due to interference of VB-P4 with the ion channels. VB-P4 did not affect the kidney, since no alteration in kidney markers (urea, creatinine) was observed due VB-P4 exposure. Histopathological studies also confirmed the liver toxicity but not the renal toxicity. VB-P4 produced in vivo cutaneous haemorrhagic lesions in rat through breakage or pore formation of capillary walls. It was also found that VB-P4 induced gastric haemorrhagic and gastric esions in the pylorus ligated rats. Histological studies also confirmed the damage of the epithelial layer of the stomach, necrotic spots. The damage is likely due to (1) direct effect of VB-P4 on the muscular epithelium (2) or through pro-oxidant effect on the stomach muscle layer.
In vitro toxicity on RBC and mast cell membrane was induced by VB-P4. It produced dose dependent haemolysis of mice RBC, thus confirming the membrane toxicity of VB-P4. The mast cells are a resident cell of several types of tissues and contain many granules rich in histamine and heparin. These mast cells play an important protective role from allergic reaction and anaphylaxis [21] as well, being intimately involved in wound healing and defense against pathogens. VB-P4 degranulated rat peritoneal mast cells and the degranulated products are capable of inducing or triggering the pathophysiology.
The present study confirmed one of the possible methods (heat treatment) to overcome the toxicity of VB-P4. Heating VB-P4 at 70- 80 °C for 20 min makes the protein free from toxicity (liver, muscle, heart, membrane). It is likely that heat treatment alters the structural configuration of VB-P4 and changes the functional/toxicity identity. Apart from heating, chelation (EDTA treatment) also makes VB-P4 free from toxicity (data not shown). This study may open a new area of snail protein induced toxicity and its remedial measure by heat treatment.
Conclusion
It may be concluded that fresh water snail flesh protein VB-P4 exhibited in vivo and in vitro toxicity which was shown on liver, GI tract, heart and cell membrane. The toxic effects of VB-P4 could be overcome by prior heat treatment. Consumption of raw snail flesh protein may be injurious to health and it would be advisable to boil/cook snail flesh before consumption to avoid the toxicity. Further studies on safe consumption of snail flesh protein are warranted.Acknowledgements
This study was partly sponsored by the Department of Science & Technology, Government of India, New Delhi, India (Ref: SR/SO/HS-58/2008). Amrita Sarkar received Junior & Senior Research fellowship from this project. Prof A Gomes received UGC-BSR fellowship award (2011-2014) of University Grants Commission, Government of India, India.References
- Prabhakar AK, Roy SP (2009) Ethno-medicinal uses of some shell fishes by people of Kosi river basin of North-Bihar, India. Ethno-Med 3: 1-4.
- Baby RL, Hasan I, Kabir KA, Naser MN (2010) Nutrient analysis of some commercially important molluscs of Bangladesh. J Sci Res 2: 390-396.
- Gauri SS, Mandal SM, Pati BR, Dey S (2011) Purification and structural characterization of a novel antibacterial peptide from Bellamya bengalensis: activity against ampicillin and chloramphenicol resistant Staphylococcus epidermidis. Peptides 32: 691-696.
- Sarkar A, Datta P, Gomes A, Das Gupta S, Gomes A (2013) Anti-osteoporosis and anti-osteoarthritis activity of fresh water snail (Viviparous bengalensis) flesh extract in experimental animal model. Open J Rheumatol Autoimmune Dis 3: 10-17.
- Sarkar A, Gomes A, Gomes A (2015) Anti-osteoporosis activity of fresh water snail (Viviparous bengalensis) flesh extracted protein fraction VB-P4 in rat models. Int J Curr Res Biosci Plant Biol 2: 60-72.
- Sarkar A, Gomes A, Gomes A (2015) Anti-osteoarthritis, anti-nociception, anti-inflammatory activities of isolated fraction of flesh extract Viviparous bengalensis in experimental model. Int J Curr Res Acad Rev 3: 66-87.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Mukherjee KL (2004) In: Medical Laboratory Technology, Tata McGraw-Hill Publishing Company Limited, New Delhi, pp. 235.
- Kondo H, Kondo S, Ikezawa H, Murata R (1960) Studies on the quantitative method for determination of haemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol 13: 43-52.
- Shay H (1945) Simple method for the uniform production of gastric ulceration in the rat. Gastroenterol 5: 43-61.
- Langendroff O (1895) Untersuchungen am uberlebenden saugethierherzen. Arch Gesamte Physiol Menschen Tiere 61: 291-332.
- Burn JH (1952) In: Practical Pharmacology. Blackwell Scientific Publications, Oxford, pp. 22
- Bulbring E (1946) Observation on the isolated phrenic nerve diaphragm preparation of the rat. Br J Pharmacol Chemother 1: 38-61.
- Rothschild AM (1965) Histamine release by bee venom phospholipase A and malatinin in the rat. Br J Pharmacol Chemother 25: 59-66.
- Lagunoff D, Benditt EP (1960) Mast cell degranulation and histamine release observed in a new in vitro system. J Exp Med 112: 571-580.
- Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, et al. (1984) Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol 174: 497-525.
- Jorgensen CR, Zimmerman TS, Wang Y (1967) Serum lactate dehydrogenase elevation in ambulatory cardiac patients. Evidence for chronic hemolysis. Circulation 35: 79-89.
- Sobel BE, Shell WE (1972) Serum enzyme determinations in the diagnosis and assessment of myocardial infarction. Circulation 45: 471-482.
- Guzy PM (1977) Creatine phosphokinase-MB (CPK-MB) and the diagnosis of myocardial infarction. West J Med 127: 455-460.
- Adams JE 3rd, Abendschein DR, Jaffe AS (1993) Biochemical markers of myocardial injury. Is MB creatine kinase the choice for the 1990s? Circulation 88: 750-763.
- Prussin C, Metcalfe DD (2003) 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 111 (2 Suppl): S486-S494.