Journal of Orthopedics & Rheumatology
Download PDF
Revision with Locking Plate and Sliding Inlay Bone Graft for Hypertrophic Nonunion after Combined Treatment of Ender Nails and Teriparatide for Atypical Femoral Stress Fracture
Research Article
Revision with Locking Plate and Sliding Inlay Bone Graft for Hypertrophic Nonunion after Combined Treatment of Ender Nails and Teriparatide for Atypical Femoral Stress Fracture
A Unique Case Report
Akira Koshiishi1, Hiroshi Hamaji1, Yasuhiro Wada1, Kimiteru Ito2, Kei Wagatsuma3, Kenji Ishii3, Masakazu Kanetaka1, Soichiro Kaneko1, Shigehito Uezono4, Naoko Shoda5, Yorito Anamizu4, Fumiaki Tokimura1, and Tsuyoshi Miyazaki1*
- 1Department of Orthopaedic Surgery, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
- 2Department of Diagnostic Radiology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
- 3Team for Neuro imaging Research, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
- 4Department of Spine Surgery, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
- 5Department of Rehabilitation, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
Address for Correspondence: Tsuyoshi Miyazaki, Department of Orthopaedic Surgery, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, 35-2 Sakaecho, Itabashi-ku, Tokyo 173-0015, Japan, Tel: 81-3-3964-1141; Fax: 81- 3-3964-1392; E-mail: miyazak14@tmig.or.jp
Citation: Koshiishi A, Hamaji H, Wada Y, Ito K, Wagatsuma K, et al. Revision with Locking Plate and Sliding Inlay Bone Graft for Hypertrophic Nonunion after Combined Treatment of Ender Nails and Teriparatide for Atypical Femoral Stress Fracture A Unique Case Report. J Orthopedics Rheumatol. 2017; 4(2): 6.
Journal of Orthopedics & Rheumatology | ISSN: 2334-2846 | Volume: 4, Issue: 2
Submission: 14 November, 2017| Accepted: 01 December, 2017 | Published: 08 December, 2017
Copyright: © Miyazaki T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction: Atypical femoral fractures (AFFs), whose characteristic radiograph patterns include beaking and the dreaded black line, are uncommon in elderly patients with osteoporosis. Bisphosphonates (BPs) are commonly prescribed for prevention of osteoporosis-related fractures. However, the long-term use of BPs has been linked to the occurrence of AFFs. Here, we report a rare case of multiple incomplete atypical stress fractures of the lateral femoral cortex with endosteal marrow edema in a patient with no history of antiresorptive treatment.
Case presentation: A 74-year-old Japanese woman sought care for severe pain in her right thigh and difficulty with ambulation. A review of the patient’s past radiograph series revealed multiple incomplete stress fractures of the lateral mid-shaft femur that had existed for at least 3 years. After progression to complete fracture induced by lowenergy trauma, we performed flexible intramedullary Ender nailing and prescribed the patient teriparatide. A positron emission tomography (PET)/computerized tomography (CT) scan with 18F-NaF revealed faint radio tracer uptake around five incomplete fracture lines in addition to marked uptake at the complete fracture site. Four months after the Ender nailing fixation operation, radiographs showed a displaced femoral stress fracture associated with breakage of three Ender nails due to hypertrophic nonunion. The patient was treated with a sliding full cortical thickness inlay bone graft combined with cancellous bone grafting and a locking plate.
Conclusion: It is important to evaluate the risk of progression from atypical stress fracture to complete fracture and to consider alternative treatment options, including surgery, in addition to conservative management with teriparatide. If standard conservative treatment following a correct diagnosis fails to produce a good outcome, a sliding full cortical thickness inlay bone graft technique with locking plate is an effective method for the difficult-to-manage AFFs.
Keywords
Atypical femoral fracture; Flexible nails; Hypertrophic nonunion; Multiple incomplete stress fractures; NaF-PET; Sliding inlay bone graft
Abbreviations
AFF: Atypical Femoral Fracture; BP: Bisphosphonate; CT: Computerized Tomography; GC: Glucocorticoid; PET: Positron Emission Tomography; PPI: Proton Pump Inhibitor; TKA: Total Knee Arthroplasty
Background
Since the first case report describing spontaneous stress fractures of the femur was published in 2005, concerns regarding the potential risk of this unusual type of fracture in patients receiving bisphosphonate (BP) therapy have developed [1]. Reports of similar fractures, which have come to be identified as ‘atypical’ in that they involve the strongest part of the femur, namely the subtrochanteric and diaphyseal region, have followed [2]. These atypical femoral fractures (AFFs) are distinct from typical osteoporotic femur fractures in that they involve a simple transverse or oblique (≤30 °) break with endosteal thickening of the lateral cortex (referred to as beaking from here onward) and the presence of generally bilateral cortical thickening of the proximal femoral shaft [2].
BPs is a commonly prescribed class of drugs for the prevention of osteoporosis-related fractures [3]. Numerous large clinical trials have demonstrated their efficacy in reducing bone turnover, increasing bone mineral density, and reducing vertebral and non-vertebral fracture risk in patients with osteoporosis [4,5]. They are generally well tolerated and considered to have an excellent safety profile even at high doses [6]. Paradoxically, AFFs have been associated recently with long-term BP therapy, though a clear causal link has not been established [7]. Nevertheless, the potential link has brought scrutiny to the widespread prophylactic use of BPs. Further complicating the question of whether BP therapy may increase risk of AFF are findings indicating that AFFs may be associated with the concomitant use of other antiresorptive agents, such as glucocorticoids (GCs) and proton pump inhibitors (PPIs) [7].
Here, we report a rare case of multiple incomplete atypical stress fractures of the lateral femoral cortex in a patient with no history of BP, GC, or PPI use. One of these stress fractures later became a complete break. The fracture was stabilized with flexible Ender nails complemented with teriparatide. Subsequent breakage of the Ender nails was attributed to hypertrophic nonunion of the AFF. The patient was treated with a sliding full cortical thickness inlay bone graft technique combined with cancellous bone grafting and locking plating.
Case Presentation
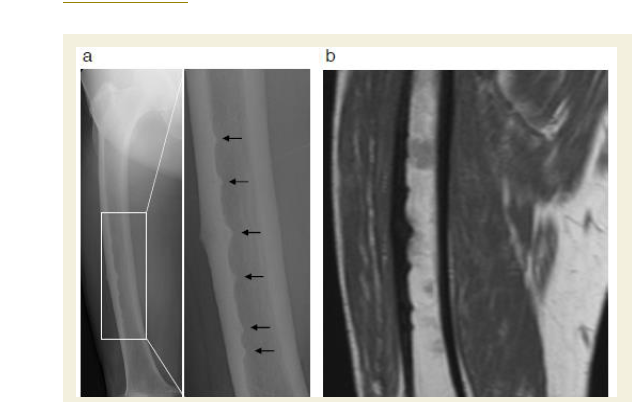
A 74-year-old Japanese woman, who had been receiving monthly oral BP (risedronate) for the past year, sought consultation in her local orthopedic clinic complaining of severe pain in her right thigh for the prior 2 weeks. Radiographs of her right femur revealed six incomplete stress fractures with beaking and a horizontal dark line (dreaded black line) of the lateral mid-shaft femur (Figure 1a). A subsequent MRI scan demonstrated marrow edema surrounding incomplete transverse fracture lines within the symptomatic femur (Figure 1b) that appeared to be typical of a BP-associated stress fractures. The patient was managed conservatively with risedronate withdrawal and limitation of weight bearing. Her severe thigh pain persisted after one month of resting her right leg. Therefore, she was referred to our hospital for further assessment.
Figure 1: Six incomplete atypical stress fractures affecting the lateral cortexof the femur.
a: Frontal femur x-ray at the time of diagnosis showing multiple incomplete stress fractures (black arrows). A higher magnification image of the framed area is shown in the right panel.
b: Coronal MRI image of the right thigh demonstrating multiple lateral corticaltriangular beakings and thickenings with endosteal marrow edema.
a: Frontal femur x-ray at the time of diagnosis showing multiple incomplete stress fractures (black arrows). A higher magnification image of the framed area is shown in the right panel.
b: Coronal MRI image of the right thigh demonstrating multiple lateral corticaltriangular beakings and thickenings with endosteal marrow edema.
A clinical evaluation of the patient in our department revealed motor weakness of her right thigh, likely consequent to her severe thigh pain. Her perception of lower extremity sensation and deep tendon reflexes seemed to be normal, and there was no muscular atrophy. Because the patient had a limping gait due to the severe pain in her right thigh, her hospitalization was scheduled in 6 days for further evaluation and a prophylactic internal fixation procedure to prevent a complete femoral fracture.
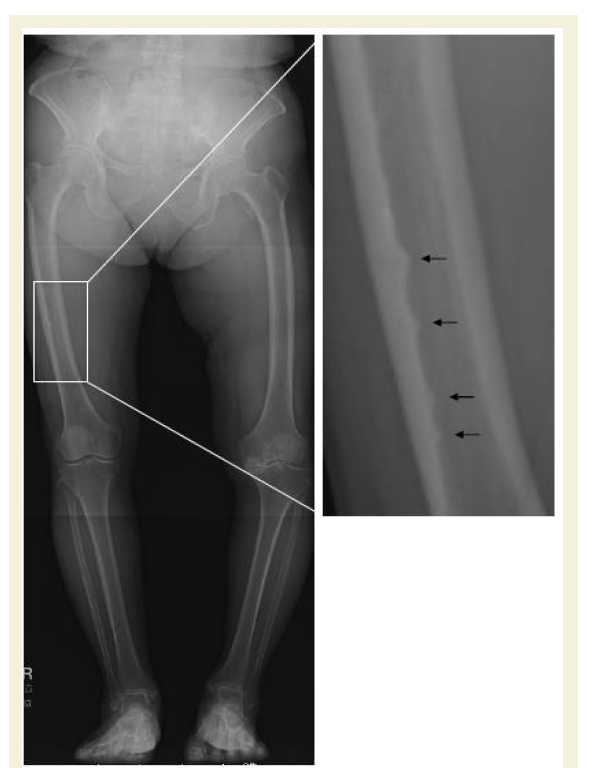
Three years prior, the patient had been treated for degenerative osteoarthritis with a primary left total knee arthroplasty (TKA), which was functioning well. While preparing for the planned fixation operation, we accessed the conventional preoperative frontal-plane radiographs of both entire lower limbs taken at the time of the left TKA. Unexpectedly, a retrospective review of the earlier radiograph showed multiple incomplete atypical fractures of the right thigh that had been missed in the initial assessment 3 years earlier (Figure 2). At least distal four beaking sites along the inner endosteal surface of the lateral femoral cortex were apparent in this earlier radiograph (Figure 2). We questioned the patient about her history of BP use and confirmed that she had never taken BP before the most recent one-year period. These findings suggest that just one-year of monthly BP may have accelerated the exacerbation of the patient’s multiple incomplete atypical femoral fractures of unknown origin.
Figure 2: Frontal x-ray image of the lower limbs taken 3 years earlier showing at least four thickening areas at the inner endosteal surface of the femur (black arrows). A higher magnification image of the framed area is shown in the right panel.
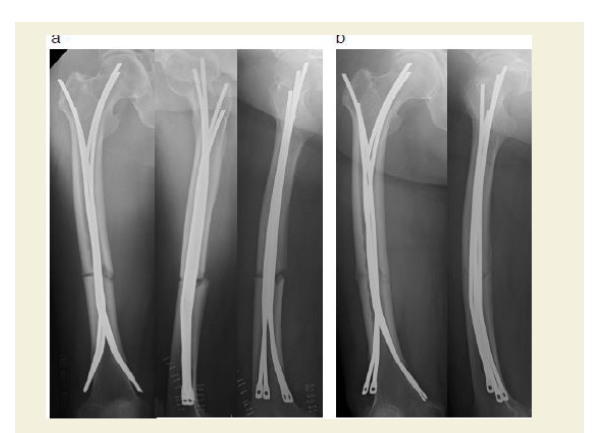
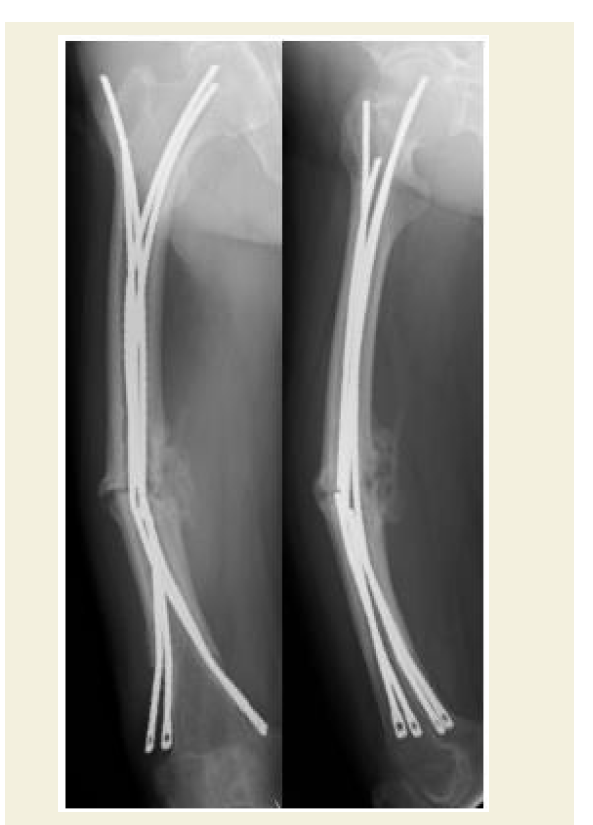
Unfortunately, while awaiting the planned fixation operation, the patient suffered a sudden fall from standing height in her home and was transferred to the emergency department of our hospital. She was admitted to the hospital one day earlier than scheduled because a radiograph demonstrated a complete femoral shaft fracture. Given the predominant hypothesis regarding AFF pathophysiology, which posits that severe suppression of bone turnover leads to an accumulation of bone microdamage and, eventually, the development of an insufficiency fracture, we sought an operative treatment that would minimize surgical dissection and damage to the periosteal vasculature while enabling micromovement at the fracture site to facilitate bone healing [1,8,9]. To stimulate callus formation and facilitate fracture healing, Ender nailing was performed and partial weight-bearing walking was started as early as possible after the operation. A slight fracture gap and rotational deformity existed at the time of operation (Figure 3a). Follow-up radiographs taken one week after the operation showed reduction of the gap and correction of the rotational deformity, likely owing to the patient’s immediate postoperative gradual weight bearing regime (Figure 3b and 4).
Figure 3: Treatment and follow-up.
a: Radiographs (anteroposterior, cross-table lateral, and frog-leg lateral) taken immediately postoperatively showing fixation of the fracture with a flexible intramedullary nail and a minimal fracture gap with rotational deformity.
b: Radiographs of the femur (anteroposterior and lateral) 1 week postoperatively, showing reduction of the gap and correction of the rotational deformity.
a: Radiographs (anteroposterior, cross-table lateral, and frog-leg lateral) taken immediately postoperatively showing fixation of the fracture with a flexible intramedullary nail and a minimal fracture gap with rotational deformity.
b: Radiographs of the femur (anteroposterior and lateral) 1 week postoperatively, showing reduction of the gap and correction of the rotational deformity.
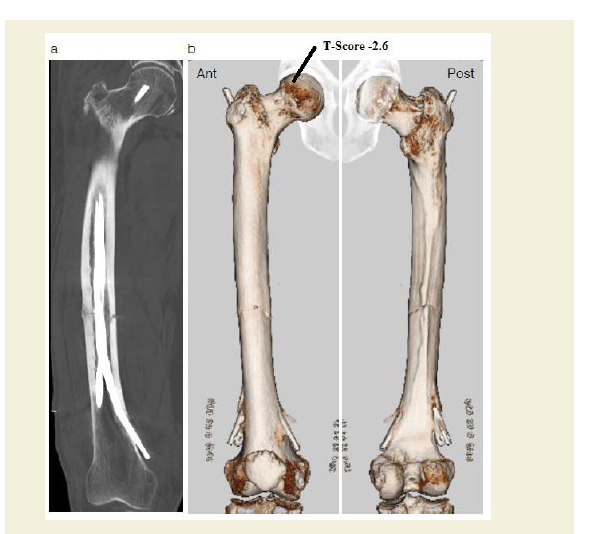
Figure 4: Postoperative 3D CT scan showing flexible intramedullary fixation with Ender nails.
a: Frontal femur x-ray at the time of diagnosis showing multiple incomplete stress fractures (black arrows). A higher magnification image of the framed area is shown in the right panel.
b: A 3D reconstruction CT scan of the femur from the anterior (Ant) and posterior (Post) aspects showing anatomic correction of the fracture gap.
a: Frontal femur x-ray at the time of diagnosis showing multiple incomplete stress fractures (black arrows). A higher magnification image of the framed area is shown in the right panel.
b: A 3D reconstruction CT scan of the femur from the anterior (Ant) and posterior (Post) aspects showing anatomic correction of the fracture gap.
Preoperative blood chemical tests were generally unremarkable: bone specific alkaline phosphatase = 8.4 mg/L (normal, 3.8-22.6); serum intact parathyroid hormone llevel = 33 pg/mL (normal, 10-65); ionized calcium level = 9.2 mg/dL (normal, 8.7-9.7); free T4 = 1.49 ng/ml (normal, 0.82-1.63); and free T3 = 2.62 pg/ml (normal, 2.10-3.80). Her 1,25 vitamin D3 levels was slightly elevated at 67 ng/ml (normal, 20-60). Her blood levels of tumor markers, including α-fetoprotein, carcinoembryonic antigen, squamous cell carcinoma antigen, and cancer antigen 19-9, were within normal limits and a myeloma screen was negative.
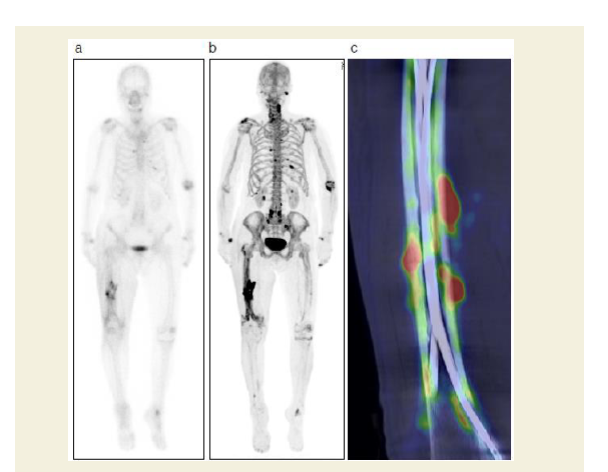
Dual energy x-ray absorptiometry conducted 1 week after the operation revealed normal bone mineral density in the femur (T-score -0.6), but osteoporosis in the lumbar spine (T-score -2.6) and forearm (T-score -3.1). Intriguingly, postoperative nuclear bone scintigraphy showed radiotracer (technetium medronic acid) up take at the fracture site as well as in distinct extraosseous uptake in the quadriceps femoris muscle (Figure 5a). Skeletal muscle uptake of bone-seeking agents may be induced by extracellular fluid expansion, enhanced regional vascularity and permeability, or elevated tissue calcium concentration. To elucidate the origin of the indistinct scintigraphy labeling, we conducted an 18F-NaF positron emission tomography (PET) complemented with computerized tomography (CT) examination of the healing thigh. Although bone scintigraphy has been the standard bone scanning method for three decades, 18F-NaF PET/CT offers an innovative advancement in bone imaging [10,11]. Notably, 18F-NaF PET/CT can distinguish complete fractures from incomplete stress fractures, whereas traditional bone scintigraphy cannot [12,13]. In the present case, 18F-NaF PET/CT demonstrated weak radiotracer uptake surrounding five incomplete stress fractures with beaking contrasted with intense uptake at the complete fracture site, indicating that NaF uptake rate may depend on fracture severity (Figures 5b and 5c). Following completion of the postoperative workup, we changed the patient’s medication prescription to a daily subcutaneous injection of parathyroid hormone and set her up with a rehabilitation program encompassing immediate gradually augmented weight-bearing.
Figure 5: NaF-PET and bone scintigraphy of complete stress fracture of the right femur after surgery.
a: Anterior and posterior 67-Ga whole-body images showing slightly increased uptake at the fracture site.
b: NaF-PET image showing massive uptake at the fracture site as well as some extraosseous uptake in the quadriceps femoris muscle.
c: Fused NaF-PET/CT coronal image demonstrating that NaF uptake was considerably lower at the sites around incomplete fracture lines than at the complete fracture site.
a: Anterior and posterior 67-Ga whole-body images showing slightly increased uptake at the fracture site.
b: NaF-PET image showing massive uptake at the fracture site as well as some extraosseous uptake in the quadriceps femoris muscle.
c: Fused NaF-PET/CT coronal image demonstrating that NaF uptake was considerably lower at the sites around incomplete fracture lines than at the complete fracture site.
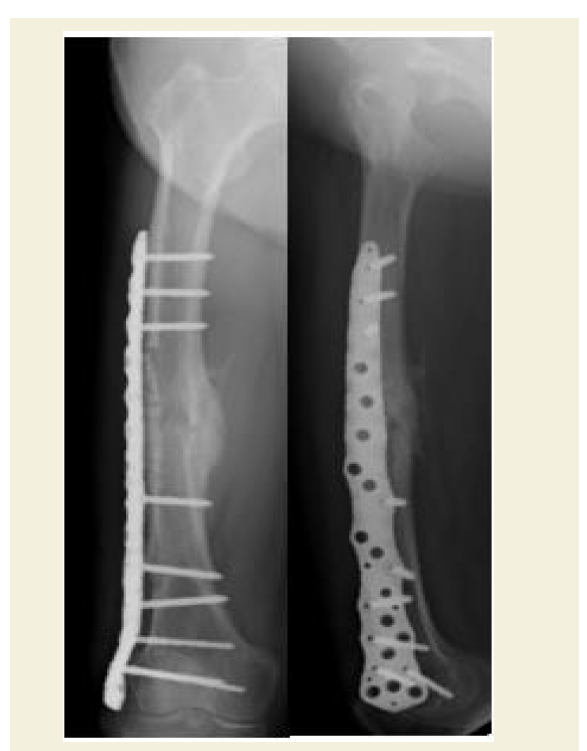
The patient’s thigh pain was not completely alleviated by the operation. Six months postoperatively, her chronic pain worsened suddenly without any predisposing trauma and she was readmitted to the hospital. Radiographs taken upon her readmission showed a slightly displaced femoral shaft fracture with breakage of three of the four nails at the original fracture site due to hypertrophic nonunion (Figure 6). The patient was re-operated with a sliding full cortical thickness inlay bone graft, as described by Albee in 1930 [14,15], combined with cancellous bone grafting. After the fracture was exposed, a full cortical thickness graft (8 cm long and 1.5 cm wide) was taken from the lateral surface of the femur and all four flexible nails were removed. The graft, which traversed the fracture site asymmetrically, was crisscrossed such that the proximal portion of the graft was placed in the distal portion of the trough and vice versa, as shown in Figure 7. The asymmetrical lengths of the graft portions guaranteed that the fracture space was covered by intact grafted bone. Following firm fitting of the graft into the trough, it was stabilized by internal fixation with a locking plate and screws (Figure 8). This procedure is advantageous in that the graft provides both internal fixation and osteogenic stimulation [16]. Starting 2 weeks after the operation, low-intensity pulsed ultrasound was applied around the fracture site for 20 minutes/day until radiographic consolidation. The patient was rehabilitated with progressive weight-bearing walking exercise. The fracture was confirmed in radiographs to be fully united 5 months following the revision surgery at which time the patient also reported alleviation of pain (Figure 8).
Figure 6: Radiographs (anteroposterior and lateral) 6 months after the Endernailing operation showed a complete fracture of right femur with evidence of nonunion and three broken flexible intramedullary nails.
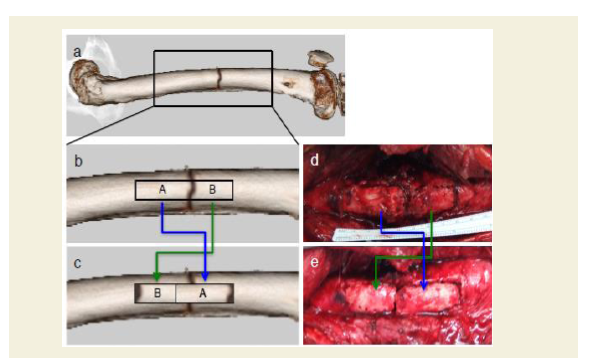
Figure 7: Modification of Albee’s bone grafting procedure.
a,b and c: Schematic representations illustrating the bone graft technique. From the lateral surface of the femur, a full cortical thickness bone graft of unequal length on either side of the fracture was taken out, and placed back into the trough.
d and e: Major surgical wound with sliding full cortical thickness inlay bone graft technique (intra operative photos).
a,b and c: Schematic representations illustrating the bone graft technique. From the lateral surface of the femur, a full cortical thickness bone graft of unequal length on either side of the fracture was taken out, and placed back into the trough.
d and e: Major surgical wound with sliding full cortical thickness inlay bone graft technique (intra operative photos).
Discussion
The etiology of AFF is thought to be suppressed bone turnover leading to microdamage accumulation in cortical bone that leaves the femur weakened and highly liable to low-impact fracture [1]. Relative to other bones, the femur may be particularly susceptible to this phenomenon due to its weight-bearing function, naturally bowed shape, and being subjected to bending forces with concomitant compression and tensile forces in the subtrochanteric and diaphyseal areas. If a patient has an undisplaced stress fracture with prodromal pain and a dreaded black line, a period of conservative therapy including partial weight-bearing and teriparatide pharmacotherapy should be considered. Surgical treatment may be indicated if conservative measures are ineffective or there is evidence of progression.
The standard procedure for surgical stabilization of an AFF is interlocking nailing [17-20]. Alternatively, Ender nailing, as was performed in this case, is a technically easier and faster procedure that omits the need for medullary canal reaming, there by preserving endosteal circulation and the blood supply to fracture segments. In addition, we preferred to use intramedullary Ender nailing in this case because the elastic nature of Ender nails allows for dynamization at the fracture site, narrowing of the fracture gap, early weight bearing, and progression of tissue differentiation. These features may improve callus formation and, ultimately, the patient’s ability to participate in activities of daily living. Six months after the operation, however, the femoral shaft refractured with breakage of three nails. The presence of a large, broad callus near the fracture gap with a radio lucent is a indicated that the refracture was due to hypertrophic nonunion.
We had hoped that Ender nailing would enable micromovement and thus support callus formation [21]. Though limited axial compressive movements can increase callus formation and accelerate fracture healing [22], too much shear stress at the fracture site can impede callus remodeling [23]. The hypertrophic nonunion in the present case was likely a result of too much multidirectional motion at the fracture site, likely due, at least in part, to early partial-weightbearing walking. The hypertrophic nonunion was corrected with autologous bone grafting and exchange of the fixation technique towards a more stable osteosynthesis. We complemented the surgical correction with ultrasound therapy, which, like external shock wave therapy, remains controversial [24,25].
PET using 18F-NaF can detect various skeletal abnormalities with higher resolution than scintigraphy. The fast kinetics of 18F-NaF enable image acquisition as early as 30 minutes post injection, improving patient compliance [10,11]. CT integration with PET provides anatomical information that can improve lesion detection. The cost of 18F-NaF and relatively undeveloped PET imaging technology may be offset by enhanced patient throughput. Our findings of differential NaF labeling in incomplete versus complete fractures indicate that NaF-PET may be a useful tool with which to identify incomplete fractures at risk of progressing to complete fracture.
Although BP therapy is considered safe and effective for fracture prevention in osteoporotic individuals [26,27], case reports have shown that long-term BP use may precipitate AFFs, such as subtrochanteric and diaphyseal femoral fractures [28-30]. It is notable that our patient had multiple atypical stress fractures for at least 3 years before she was seen in our department, years before she started BP therapy and with no history of GC or PPI use. Her case suggests that some patients who sustain an AFF may have had predisposing conditions prior to initiation of BP therapy, though subsequent longterm BP therapy may contribute ultimately to the AFF pathogenesis. Given that BP clearance from the bone matrix and the return to normal bone remodeling may take more than 7 years [31,32], a BP holiday to avoid AFF formation remains enigmatic. Further investigation into the origin of AFFs is required to demonstrate the specific effects of BPs on cortical bone quality, tissue properties, and their effects on the mechanical performance of the skeleton.
Conclusion
Conservative treatment of a correctly diagnosed AFFs, which includes limitation of weight bearing, discontinuation of BP therapy, teriparatide therapy, and, if needed, fracture stabilization, may be insufficient for a good outcome. Better methods for accurate detection of progression from an incomplete stress fracture to a complete fracture are needed. NaF-PET can be a useful tool for detecting signs of such a risk. The growing body of case reports describing various AFF cases that were managed successfully provides a useful resource for guiding treatment decisions for these difficult-to-manage injuries.
References
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, et al. (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90: 1294-1301.
- Lenart BA, Lorich DG, Lane JM (2008) Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 358: 1304-1306.
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG (2008) Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 22: 346-350.
- Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, et al. (2002) Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 23: 517-523.
- Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, et al. (2002) Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 23: 508-516.
- Dunstan CR, Felsenberg D, Seibel MJ (2007) Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. Nat Clin Pract Oncol 4: 42-55.
- Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, et al (2010) Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 25: 2267-2294.
- Ng YH, Gino PD, Lingaraj K, Das De S (2011) Femoral shaft fractures in the elderly--role of prior bisphosphonate therapy. Injury 42: 702-706.
- Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, et al. (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 89: 349-353.
- Hoh CK, Hawkins RA, Dahlbom M, Glaspy JA, Seeger LL, et al. (1993) Whole body skeletal imaging with [18F] fluoride ion and PET. J Comput Assist Tomogr 17: 34-41.
- Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, et al. (1992) Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med 33: 633-642.
- Oh Y, Wakabayashi Y, Kurosa Y, Ishizuki M, Okawa A (2014) Stress fracture of the bowed femoral shaft is another cause of atypical femoral fracture in elderly Japanese: a case series. J Orthop Sci 19: 579-586.
- Kim HS, Jung HY, Kim MO, Joa KL, Kim YJ, et al. (2015) Successful conservative treatment: multiple atypical fractures in osteoporotic patients after bisphosphate medication: a unique case report. Medicine (Baltimore) 94: e446.
- Albee FH (1930) Principles of the treatment of non-union of fracture. Surg Gynecol Obstet: 289-320.
- Hansson G, Jerre R, Markhede G, Andersson GB (1992) Treatment of non-union after tibial shaft fracture with a full cortical thickness inlay bone graft. Ups J Med Sci 97: 169-176.
- Hohl M (1965) Treatment of ununited fractures of the long bones; surgical treatment and technique. J Bone Joint Surg Am 47: 179-190.
- Banffy MB, Vrahas MS, Ready JE, Abraham JA (2011) Nonoperative versus prophylactic treatment of bisphosphonate-associated femoral stress fractures. Clin Orthop Relat Res 469: 2028-2034.
- Capeci CM, Tejwani NC (2009) Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am 91: 2556-2561.
- Ward WG Sr, Carter CJ, Wilson SC, Emory CL (2012) Femoral stress fractures associated with long-term bisphosphonate treatment. Clin Orthop Relat Res 470: 759-765.
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH (2010) Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 468: 3393-3398.
- Yamaji T, Ando K, Nakamura T, Washimi O, Terada N, et al. (2002) Femoral shaft fracture callus formation after intramedullary nailing: a comparison of interlocking and Ender nailing. J Orthop Sci 7: 472-476.
- Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, et al. (1998) Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res: S132-S147.
- Augat P, Burger J, Schorlemmer S, Henke T, Peraus M, et al. (2003) Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res 21: 1011-1017.
- Romano CL, Romano D, Logoluso N (2009) Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol 35: 529-536.
- Bashardoust Tajali S, Houghton P, MacDermid JC, Grewal R (2012) Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am J Phys Med Rehabil 91: 349-367.
- Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, et al. (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med 333: 1437-1443.
- Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1799-1809.
- Isaacs JD, Shidiak L, Harris IA, Szomor ZL (2010) Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res 468: 3384-3392.
- Schilcher J, Michaelsson K, Aspenberg P (2011) Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 364: 1728-1737.
- Bauer DC (2012) Atypical femoral fracture risk in patients treated with bisphosphonates. Arch Intern Med 172: 936-937.
- Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, et al. (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38: 617-627.
- Ott SM (2011) What is the optimal duration of bisphosphonate therapy? Cleve Clin J Med 78: 619-630.