Journal of Ocular Biology
Levels of Plasma Homocysteine,Serum Vitamin B12 and Folic Acid in Primary Open Angle Glaucoma - A Case Control Study
A. Yadav*, V. Singh, S.K. Bhasker, V. Katiyar and A.A. Mehdi
- 1 Department of Ophthalmology, King George’s Medical University,Lucknow, Uttar Pradesh, India
*Address for Correspondence: A. Yadav, Department of Ophthalmology, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India, Tel: +918874874333; Fax: +91 522 2257539; E-mail: ankuryd@gmail.com
Citation: Yadav A, Singh V, Bhasker SK, Katiyar V, Mehdi AA. Levels of Plasma Homocysteine, Serum Vitamin B12 and Folic Acid in Primary Open Angle Glaucoma - A Case Control Study. J Ocular Biol. 2016;4(1): 5.
Copyright & copy; © 2016 Yadav A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 4, Issue: 1
Submission: 27 October, 2016 | Accepted: 28 November, 2016 | Published: 05 December, 2016
Abstract
Purpose: To study the levels of Plasma homocysteine and serum vitamin B12 and folic acid in cases of Primary Open Angle Glaucoma(POAG).
Methods: It was a case-control cross sectional observational study for duration of one year. Calculated sample size was 28. The cases were randomly selected among all POAG patients >=40 yrs attending glaucoma clinic. Age and sex matched healthy individuals were recruited in the control group. Assay of homocysteine was carried out using the Human homocysteine, serum vitamin B12 and folic acid was carried out in both the groups statistical analysis was done using SPSS 20.0 version. The results were presented in mean ± SD and percentages. Unpaired t-test was used to compare the study parameters between cases and controls (CI= 95%; p<0.05significant). Pearson Correlation coefficient was used to find the correlation among the study parameters. Linear regression analysis was carried out to find the strength of the correlation between the study parameters.
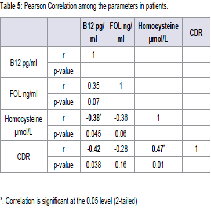
Results: Level of Vitamin B12 was significantly lower and homocysteine was significantly higher in the patients of advanced glaucoma than moderate and mild. There was poor negative correlation between vitamin B12 and homocysteine, vitamin B12 and Cup Disc Ratio (CDR). There was significant moderate correlationbetween CDR and homocysteine (r=0.47, p=0.01).
Conclusions: Elevated homocysteine level may act as a pathological link on the progression of POAG. Understanding and characterizing its role in the pathogenesis POAG of could help in identifying novel target to combat this blinding disease which is the major cause of blindness in adults.
Keywords
Primary open angle glaucoma; Homocysteine; Vitamin B12; Folic acid
Introduction
Glaucoma is one of the prominent causes of blindness. Elevated intraocular pressure is the most important risk factor. However recent evidences indicate that vascular or neurotoxic factors may also play a role in the pathophysiology of Primary Open Angle Glaucoma(POAG). Apoptosis of retinal ganglion cell has been observed either due to impaired blood supply to the head of optic nerve or direct toxic action of various cytotoxic agents including Hcy [1,2].
A direct dose response association between hyperhomocysteinemia with vascular disease has already been reported [3,4]. Risk is mainly due to the inhibition of the endothelial synthesis of nitric oxide (NO) by asymmetric dimethylarginine which is increased by elevated hcy. Hence, chronic elevation of plasma hcy impairs endothelium dependent NO mediated vasodilatation. Treatment with folate and B12 reduces Hcy levels in subjects with or without any defect in the enzymes involved in its metabolism [5,6]. Willems et al. reported that supplementation with these vitamins can improve vascular function in hyperhomocysteinemic patients with evident coronary artery disease [7].
Previous prospective studies reported significant high level of Hcy in patients with pseudoexfoliative glaucoma (PEXG) [8-11]. The mean serum folic acid level was found to be significantly low in subjects with PEXG. The increased risk of vascular disease among patients with PEXG glaucoma can be explained by the impaired endothelium dependent vasodilatation by the elevated Hcy [12]. Although the positive correlation between the level of Hcy and PEXG glaucoma has been reported, the role of homocysteine (hcy) in context with POAG, has yielded mixed results [13-15]. Low folate, vitamin-B12 status, or renal impairment account for the majority of cases where increased levels of hcy are observed. Differences in these factors could, in part, account for the disparity in reported results. Besides, its prevalence in Indian patients with glaucoma or its correlation with the level of folate and B12 has not yet been established.
Based on the hypothesis that vascular dysfunction is one of the factors involved in the pathogenesis of glaucoma and Vitamin B12, folate are the two most important dietary factors that influence Hcy metabolism, their serum level were studied in several POAG cohorts and compared with the controls.
Materials and Methods
The study was conducted under the tenets of declaration of Helsinski. It was a tertiary centre based cross sectional, case-control, observational study for duration of one year from August 2015-July 2016. The sample size was calculated taking the prevalence of POAG among >= 40 yrs of age population as 1.7%, based on the results of two landmark studies from India and confidence interval taken as 95% [16,17]. Calculated sample size was 28. The cases were randomly selected among all POAG patients >=40 yrs attending glaucoma clinic of Department of Ophthalmology. POAG was defined by the presence of an open-angle, Intraocular Pressure (IOP) higher than 21 mmHg, typical glaucomatous cupping, and visual field defects in at least one eye in two consecutive visits. Age and sex matched healthy individuals coming to ophthalmology Outpatient department (OPD) for refraction was recruited in the control group. An informed voluntary consent was taken from all the cases and controls.
Patients in both the groups were screened to rule out any exclusion criteria. Detailed clinical history followed by general and systemic evaluation to identify the patients with risk factors for vascular disease such as hypertension, diabetes mellitus, hyperlipidemia, cardiovascular, and cerebrovascular diseases. Patients on medication like anticonvulsants and immunosuppressives, hormone substitutes, cholesterol-lowering agents, antidepressants, vitamin supplements were excluded. Any subject with a history of recent cataract surgery <3 months, previous intravitreal injections/laser, retinal venous occlusive disease, age Related Macular degeneration (ARMD) was excluded. History to rule out any surgery under General Anaesthesiain last 3 months, pregnancy, chronic alcohol abuse was taken.
The patient was evaluated under the following heads: Best corrected visual acuity (LogMAR), Slit lamp biomicroscopy, IOP measurement employing Goldmann applanation tonometry, Cup disc ratio (CDR) by Slit lamp biomicroscopy using 78D, Gonioscopy, Fundoscopy using technique of indirect ophthalmoscopy, Visual fieldexamination (HVF 30-2). The patients were divided on the basis of severity, based on ‘Hodapp classification’ [18].
Sample collection and preparation
Patient was sent to the ‘Department of Biochemistry’ for the sample retrieval. Blood sample of the patient was drawn by vein puncture by the lab technician using 5ml metal-free plastic syringe fitted with a 24 Gauge stainless steel needle (Nirlife, one use, manufactured by NIRMA LIMITED, Sachana Gujarat India) under contamination controlled conditions and blood samples were collected in 4 ml VACUTAINER (VAKU-8, Hindustan Syringes and Medical Devices Limited manufactured in Faridabad INDIA). Lab technician was masked during the collection of sample. The volume of the samples ranged from 7 ml to 8 ml. Blood was transferred in to glass tubes for separation of serum. The tubes containing blood were set on to the stand and left for 30 minutes to allow the blood to clot. Soon after, the samples were centrifuged at 1000 x g for 10 minutes and serum was carefully removed in to another tube.
Assay of serum folic acid, vitamin B12 was done using elecsys Folate III assay method which employs a competitive test principle using natural folate binding protein (FBP) specific for folate, intrinsic factor specific for vitamin B12, respectively. Assay of homocysteine was carried out using the Human homocysteine ELISA kit (My BioSource, Inc. San Diego, California, USA).
Statistical analysis
All the analysis was carried out by using SPSS 20.0 version(Chicago, Inc., USA). The results were presented in mean ± SD and percentages. Unpaired t-test was used to compare the study parameters between patients and controls. Pearson Correlation coefficient (r) was used to find the correlation among the study parameters. Linear regression analysis was carried out to find the strength of the correlation between the study parameters. Confidence Level was taken as 95% and p<0.05 was considered significant.
Results
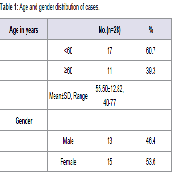
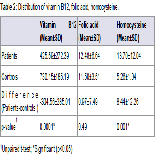
A total of 28 patients were included in the study. The cases fulfilled the inclusion and exclusion criteria. Equal number of age and sex matched controls not having glaucoma or any other disease affecting the levels of Hcy, vitamin B12 and folic acid. As the study and control group of our study showed no statistically significant difference in age and sex and thus they did not influence the outcome/ measures of the study. Of the total no. of cases (28), 60.7% of the patients were <60 yrs of age. The mean age of the patients was 55.50 (±12.82) yrs (range= 40- 77 yrs ). More than half (53.6) of the patients were females (Table 1). The Vitamin B12 level was significantly lower among the patients (425.59±272.39) compared to normal patients(730.15±185.19) (p<0.05). The folic acid level was higher among the patients (12.48±6.64) than normal (11.50±3.51), but the difference was statistically not significant (p>0.05). Homocysteine level was significantly higher among the patients (13.70±12.04) than normal(p<0.05) (Table 2).
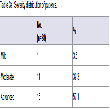
The CDR was similar in both the right and left side with no asymmetrical cupping. The advanced glaucoma level was in 57.1% and moderate was in 39.3%. However, mild glaucoma was observed in 3.6% of the patients. On comparing parameters among the patients according to glaucoma severity, vitamin B12 was significantly lower and homocysteine was significantly higher in the patients of advanced glaucoma than moderate and mild. There was no significant difference in the level of folic acid, among the different severity groups of glaucoma (Table 3a and 3b).
There was no significant (p>0.05) difference in the Vitamin B12, folic acid, homocysteine between the age group of <60 and ≥60 yrs. Also, no significant (p>0.05) difference in the Vitamin B12, folic acid, homocysteine and CDR between male and females was observed (Table 4a and 4b).
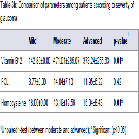
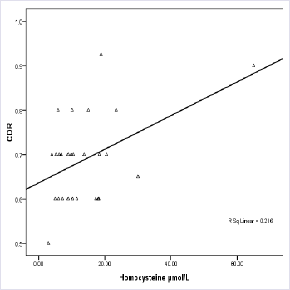
There was significant moderate correlation between CDR and homocysteine (r=0.47, p<0.05). Also, there was poor negative correlation between vitamin B12 and homocysteine, vitamin B12 level and CDR. Folic acid level was poorly correlated with Vitamin B12, Hcy level and CDR (Table 5)Figure 1.
Discussion
Elevated intraocular pressure has been the most relevant risk factor, but vascular, or neurotoxic factors have also been implicated in the pathophysiology of POAG [19]. The independent role of IOP alone is in doubt. Glaucoma can occur at any IOP level; furthermore, elevated IOP or ‘ocular hypertension’ is usually sustained without damage [20].
The effects of elevated Hcy levels on retinal function in both, in vitro and in vivo models have shown that Hcy induces apoptosis in retinal ganglion cells [21]. Several studies have demonstrated that neuronal cells die by apoptosis as the expression of Bax (Bcl-2- associated X protein), a pro-apoptotic protein, is found in increased levels in glaucoma damage [22]. Studies have also demonstrated that the neurotoxic effects of homocysteine are also associated with an activation of type II metabotropic glutamate receptors [23].
The result of raised Hcy in POAG in the present study is consistent with the few previous studies. Bleich et al. for the first time found that Hcy levels and the frequency of heterozygous ethylenetetrahydrofolate reductase (MTHFR) C677T mutation are increased in open-angle glaucoma [24]. Clement et al. in their study “Plasma hcy, MTHFR gene mutation and Open angle Glaucoma” found that the mean plasma homocysteine level was significantly higher in POAG, pseudoexfoliation glaucoma (PXG), normal tension glaucoma (NTG) [25]. It was a case control study. There was no significant difference in frequency of MTHFR C677T gene mutation between groups. This suggested that MTHFR C677T mutation might not be the cause of Hcy elevation. Roedl et al. in the prospective casecontrol study found that patients with POAG had significantly higher mean Hcy levels both in tear fluid and plasma (p<0.05) than in control subjects [26]. Hcy level in tear fluid was significantly correlated with plasma Hcy level in POAG patients (p<0.05), but not in controls (p> 0.05). None of the above studies showed any association between Hcy level and B12 vitamin status in subjects with POAG.
In contrast to the above results, Wang et al. in their study “total plasma homocysteine in POAG” found no significant plasma homocysteine level among POAG patients and normal control individual [27]. Altintas et al. found that the mean plasma homocysteine level showed statistically significant elevation in the pseudoexfoliation syndrome (p<0.05) and the pseudoexfoliation glaucoma (PEXG) (p<0.05) groups but not in the POAG group (p>0.05) when compared with the control group [28]. Similarly, Cumurcu et al. in their case control study to assess serum homocysteine, vitamin B12 and folic acid levels in different types of glaucoma found statistically significant elevated levels of homocysteine in Pseudoexfoliation glaucoma only [29].
The present study, for the first time, provides association between the levels of vitamin B12 and Hcy and their correlation with the severity of POAG. The Vitamin B12 level was significantly lower and homocysteine level was significantly higher in the patients with advanced glaucoma than moderate and mild (Table 5). The folic acid level showed no significant differences in the study groups. Also Folic acid level was poorly correlated with Vitamin B12 level. This can be attributed to the fact that a substantial proportion of the population of India adheres to a vegetarian diet for cultural and religious reasons. Even the food consumed by non vegetarian Indians usually contains less animal derived protein than in the typical western diet [30]. Also, vitamin B12 deficiency is expected to be higher in elder Indians owing to high prevalence of Helicobacter pylori infection. The atrophic gastritis may also be implicated in the impaired B12 adsorption in the elderly population [31]. Thus, Hyperhomocysteinemia in Indians living in India is more attributable to low concentrations of vitamin B12 [32]. This study failed to take the dietary habit of the subjects. Sato et al. reported the effect of folate and B12 in Japanese patients with hyperhomocysteinemia [33]. Folic acid and B12 alone was shown to reduce Hcy levels by 22 and 11%, respectively. But both can act synergistically to cause a reduction of 38.5% in the Hcy levels. However, a general recommendation for supplementing folate, B12 and other nutritional vitamins in elder subjects is outside the scope of this study.
Increased Hcy level show a statistically significant positive correlation with the CDR suggesting more important role of homocysteine in the pathology of POAG than Vitamin B12 (Table 5 and Figure 1). An elevation of Hcy level may cause changes in the microvasculature in the optic nerve head and impair optic nerve blood flow and ocular vasculopathy via a vasoconstrictive effect, endothelial injury, smooth muscle proliferation, platelet activation, thrombogenesis, and apoptotic cell death in retinal ganglion cells [34,35].
A multi-centric population based longitudinal study is further warranted for the establishment of relation between Hcy with glaucoma in our population. Understanding and characterizing the role of homocysteine in the pathogenesis of POAG could help in identifying novel target to combat this blinding disease which is the major cause of blindness in adults. The results of this study suggest the necessity of measuring the serum levels of Hcy, B12 and folic acid periodically in elderly subjects with glaucoma that may help to prevent further optic vasculopathy.
References
- Feldman F, Sweeney VP, Drance SM (1969) Cerebro-vascular studies in chronic simple glaucoma. Can J Ophthalmol 4: 358-364.
- Broadway DC, Drance SM (1998) Glaucoma and vasospasm. Br J Ophthalmol 82: 862-870.
- Stampher MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, et al. (1992) A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 268: 877-881.
- Selhub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, et al. (1995) Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med 332: 286-291.
- Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, et al. (2004) Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry 12: 631-638.
- Omran ML, Morley JE (2000) Assessment of protein energy malnutrition in older persons, part 1: History, examination, body composition and screening tools. Nutrition 16: 50-63.
- Willems FF, Aengevaeren WR, Boers GH, Blom HJ, Verheugt FW (2002) Coronary endothelial function in hyperhomocysteinemia: improvement after treatment with folic acid and cobalamin in patients with coronary artery disease. J Am Coll Cardiol 40: 766-772.
- Bleich S, Jünemann A, Von Ahsen N, Lausen B, Ritter K, et al. (2002) Homocysteine and risk of open angle glaucoma. J Neural Transm (Vienna) 109:1499-1504.
- Leibovitch I, Kurtz S, Shemesh G, Goldstein M, Sela BA, et al. (2003) Hyperhomocysteinemia in pseudoexfoliation glaucoma. J Glaucoma 12: 36-39.
- Wang G, Medeiros FA, Barshop BA, Weinreb RN (2004) Total plasma homocysteine and primary open-angle glaucoma. Am J Ophthalmol 137: 401-406.
- Cumurcu T, Sahin S, Aydin E (2006) Serum homocysteine, vitamin B 12 and folic acid levels in different types of glaucoma. BMC Ophthalmol 6: 6.
- Hankey GJ, Eikelboom JW (1999) Homocysteine and vascular disease. Lancet 354: 407-413
- Türkcü FM, Köz OG, Yarangümeli A, Oner V, Kural G (2013) Plasma homocysteine, folic acid, and vitamin B₁₂ levels in patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and normotensive glaucoma. Medicina (Kaunas) 49: 214-218.
- Xu F, Zhao X, Zeng SM, Li L, Zhong HB, et al. (2012) Homocysteine, B vitamins, methylenetetrahydrofolate reductase gene, and risk of primary open-angle glaucoma: a meta-analysis. Ophthalmology 119: 2493-2499.
- Ghanem AA, Mady SM, El-Awady HE, Arafa LF (2012) Homocysteine and hydroxyproline levels in patients with primary open-angle glaucoma. Curr Eye Res 37: 712-718.
- Dandona L, Dandona R, Mandal P, Srinivas M, John RK, et al. (2000) Angle-closure glaucoma in an urban population in Southern India: The Andhra Pradesh eye disease study. Ophthalmology 107: 1710-1716.
- Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, et al. (2003) Glaucoma in a rural population of southern India: the Aravind comprehensive eye survey. Ophthalmology 110: 1484-1490.
- Sponsel WE, Arango S, Trigo Y, Mensah J (1998) Clinical classification of glaucomatous visual field loss by frequency doubling perimetry. Am J Ophthalmol 125: 830-836.
- Moore P, El-sherbeny A, Roon P, Schoenlein PV, Ganapathy V, et al. (2001) Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp Eye Res 73: 45-57.
- Harder JM, Fernandes KA, Libby RT (2012) The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep 2: 530.
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, et al. (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A 94: 5923-5928.
- Harder JM, Fernandes KA, Libby RT (2012) The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep 2: 530.
- Ho PI, Ortiz D, Rogers E, Shea TB (2002) Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res 70: 694-702.
- Bleich S, Jünemann A, Von Ahsen N, Lausen B, Ritter K, et al. (2002) Homocysteine and risk of open-angle glaucoma. J Neural Transm (Vienna) 109: 1499-1504.
- Clement CI, Goldberg I, Heale PR, Graham SL (2009) Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma 18: 73-78.
- Roedl JB, Bleich S, Reulbach U, Rejdak R, Kornhuber J, et al. (2007) Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma 16: 234-239.
- Wang G, Medeiros FA, Barshop BA, Weinreb RN (2004) Total plasma homocysteine and primary open-angle glaucoma. Am J Ophthalmol 137: 401-406.
- Altintaş Ö, Maral H, Yüksel N, Karabaş VL, Dillioğlugil MÖ, et al. (2005) Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 243: 677-683.
- Cumurcu T, Sahin S, Aydin E (2006) Serum homocysteine, vitamin B 12 and folic acid levels in different types of glaucoma. BMC ophthalmol 6: 6.
- Price SR (1984) Observations on dietary practices in India. Hum Nutr Appl Nutr 38: 383-389.
- Refai W, Seidner DL (1999) Nutrition in the elderly. Clin Geriatr Med 15: 607-625.
- Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, et al. (2006) Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India 54: 775-782.
- Finkelstein JD (1998) The matabolism of homocysteine: pathways and regulation. Eur J Pediatr 157 Suppl 2: S40-S44.
- Zaidi T, Zaidi T, Yoong P, Pier GB (2013) Staphylococcus aureus corneal infections: effect of the panton-valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest Ophthalmol Vis Sci 54: 4430-4438.
- Hankey GJ, Eikelbom JW (2001) Homocysteine and stroke. Curr opin Neurol 14: 95-102.