Journal of Oral Biology
Download PDF
Research Article
Antimicrobial Activity of Sophora Japonica Extract Against Oral Bacteria
Su-Mi Cha1, Jeong-Dan Cha2, Soon-Il Yun2,5*, Eun-Jin Jang3 and Sung-Mi Choi4,5
- 1Department of Oral Microbiology and Institute of Oral Bioscience, Chonbuk National University, Jeonju, Republic of Korea
- 2Department of Food Science & Technology, College of Agriculture & Life Sciences, Chonbuk National University, Jeonju, Republic of Korea
- 3Department of Dental Technology, Daegu Health College, Daegu, South Korea
- 4Department of Dental Hygiene, Daegu Health College, Daegu, South Korea
- 5Department of Dental Hygiene, Daegu Health College, Daegu, South Korea
*Address for Correspondence: Soon-Il Yun, Department of Food Science & Technology, College of Agriculture & Life Sciences, Chonbuk National University, 664-14 Duckjin-Dong, Duckjin-Ku, Jeonju, Chonbuk,561-756 Republic of Korea, Tel: +82-63-270-2566; Fax: +82-63-270-4049; E-mail: siyun@jbnu.ac.kr
Citation: Cha SM, Cha JD, Yun SI, Jang EJ, Choi SM. Antimicrobial Activity of Sophora Japonica Extract Against Oral Bacteria. J Oral Biol. 2017; 4(2): 9.
Copyright: © 2017 Cha SM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 4, Issue: 1
Submission: 12 October, 2017 | Accepted: 28 November, 2017 | Published: 07 December, 2017
Keywords
Sophora japonica; Antibacterial activity; Oral pathogen; Biofilm formation; Synergistic effect; Minimum inhibitory concentrations (MICs); Minimum bactericidal concentrations (MBCs)
Abstract
Background: The flower and flower buds of Sophora japonica L. have the same medicinal uses, with significant biological activity, in the treatment of bleeding hemorrhoids, hematuria, hematemesis, hemorrhinia, uterine or intestinal hemorrhage, metrorrhagia, leukorrhea, conjunctivitis, pyoderma, arteriosclerosis, hypertension, and dizziness.
Methods: This study aimed to investigate the synergistic antibacterial activity with existing antimicrobial agents against oral pathogen. The synergistic effects and anti biofilm of 50% ethanol extract of Sophora japonica (SJEE) were evaluated against oral bacteria, either alone or with antibiotics, via broth microdilution method, time-kill method, and crystal violet assay.
Results: MIC/MBC values for SJEE, Ampicillin, Gentamicin, Erythromycin,and Vancomycin against all the tested bacteria ranged between 0.125-4/0.5-16 mg/mL, 0.0313-16/0.125-32 μg/mL, 2-256/4-512 μg/mL, 0.008- 32/0.016-64 μg/mL, and 0.25-64/1-128 μg/mL, respectively. The synergistic effects were exhibited on SJEE with antibiotics against oral bacteria at Fractional Inhibitory Concentration Index (FICI)<0.5. Moreover, SJEE and antibiotics were found to synergistically reduce biofilm formation. 1-6 hours of treatment with 1/2 MIC of SJEE with 1/2 MIC of antibiotics resulted from an increase of the rate of killing in units of CFU/mL to a greater degree than was observed with alone.
Conclusion: The SJEE was exerted a strong bactericidal effect in drug combinations against oral bacteria and biofilm formation.
Abbrevations
SJEE: Sophora japonica Ethanol Extract; MICs: Minimum Inhibitory Concentrations; MBCs: Minimum Bactericidal Concentrations; CFU: Colony Forming Unit; FIC index: Fractional Inhibitory Concentration; FBC Index: Fractional Bactericidal Concentration Index
Introduction
More than 700 different bacterial species have been detected in the oral cavity of humans [1]. Saliva contains 108 to 109 bacteria per milliliter, and some of these adhere to the teeth and initiate formation of a dental biofilm, previously called dental plaque [2,3]. Dental caries are caused by demineralization of the enamel of the tooth by acid produced from dietary sugars by micro-organisms growing as a biofilm or plaque [4,5]. Until a few decades ago, development of caries was ascribed to a few gram-positive bacterial species in the biofilm, i.e. the specificbiofilm/plaque hypothesis, and Streptococcus mutans, Streptococcus sobrinus together with some Lactobacillus species were regarded as key pathogens [6]. While especially S. mutans, Actinomyces, and Lactobacillus species were previously regarded as responsible for caries, the list of caries-associated bacteria now includes species of the genera Actinomyces, Lactobacillus, Dialister, Eubacterium, Olsenella, Bifidobacterium, Atopobium, Propionibacterium, Scardovir, Abiotrophia, Selenomonas, and Veillonella in addition to carbohydrate fermenting oral streptococci [6,7].
Periodontitis is characterized by inflammation of the periodontal tissues. Generally, the etiological agents of periodontal diseases are Gram-negative rods including Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Prevotella, Fusobacterium, and Porphyromonas gingivalis [8,9]. Recent reports have suggested a potential role for periodontal infections in more serious systemic diseases including cardiovascular disease, respiratory infections, and diabetes, which are pathologies that significantly affect the overall health of the infectedindividual [10-12].
Mechanical dental plaque removal is an efficient procedure to prevent periodontitis and caries. However, the use of chemical compounds as a complementary method is also necessary and has proven to be a valuable tool to decrease tooth biofilm formation. Natural products are a major source of new natural drugs and their use as an alternative medicine for treatment of various diseases has been increased in the last few decades [13]. Sophora japonica L. is a tree native to Korea, China, and Japan. Both the flower and flower buds have the same medicinal uses, with significant biological activity, in the treatment of bleeding hemorrhoids, hematuria, hematemesis, hemorrhinia, uterine or intestinal hemorrhage, metrorrhagia, leukorrhea, conjunctivitis, pyoderma, arteriosclerosis, hypertension, and dizziness [14-16]. Flavones from the buds and pericarp were discovered as haemostatic constituents [17,18]. Triterpenes, phospholipids, alkaloids, amino acids and fatty acids have been reported as the main chemical constituents of the seeds of this plant [19,20]. Among these compounds, kaempferol, quercetin, rutin, isorhamnetin, genistein, and sophoricoside are the major active constituents of S. japonica [15,17-20]. Rutin, in particular, is the most important and abundant constituent of S. japonica [15]. Crude extracts in vitro, the ethanol extract from flower buds of S. japonica exhibits a significant antibacterial activity against Staphylococcus aureus, Propionibacterium avidum, and Propionibacterium acnes under weak acidic conditions [21]. The Ethyl acetate (EtOAc)- soluble fraction is effective in inhibiting Escherichia coli, Klebsiella pneumoniae, and S. aureus [22].
In this study, the antimicrobial activities of 50% ethanol extract of Sophora japonica (SJEE) against oral bacteria were assessed using broth microdilution method, time kill method, and crystal violet assay for synergistic effect and biofilm formation of the combination with antibiotics.
Materials and Methods
Plant material and preparation of 50% ethanol extract of Sophora japonica (SJEE)
Dried flowers from S. japonica (2 kg) were macerated and extracted three times with 50% EtOH (10 L) for 4 h at 80 °C. The combined 50% EtOH extract (30 L) was clarified by filtration and evaporated to obtain a dark brown syrup (200 g). One hundred mg/ mL of extract was dissolved in 10% dimethyl sulfoxide (DMSO, Sigma Chemical Co., St. Louis, MO, USA) and then, diluted with bacteria culture medium for testing. All of the extract was kept at 4 °C in the dark until further use.
Bacterial strains
The oral bacterial strains used in this study were: Streptococcus mutans ATCC 25175 (American Type Culture Collection), Streptococcus sanguinis ATCC 10556, Streptococcus parasanguinis KCOM 1497 (Korean Collection for Oral Microbiology), Streptococcus sobrinus ATCC 27607, Streptococcus ratti KCTC (Korean Collection for type cultures) 3294, Streptococcus criceti KCTC 3292, Streptococcus downei KCOM 1165, Streptococcus anginosus ATCC 31412, Streptococcus gordonii ATCC 10558, Aggregatibacter actinomycetemcomitans ATCC 43717, Fusobacterium nucleatum ATCC 10953, Prevotella intermedia ATCC 25611, and Porphylomonas gingivalis ATCC 33277. Brain-Heart Infusion (Difco Laboratories, Detroit, MI) broth supplemented with 1% yeast extract (Difco) was used for all bacterial strains except P. intermedia and P. gingivalis. For P. intermedia and P. gingivalis, BHI broth containing hemin 1 μg/mL (Sigma) and menadione 1 μg/mL (Sigma) was used.
Minimum inhibitory concentrations/minimum bactericidal concentrations assay
The minimum Inhibitory Concentrations (MICs) were determined for 50% ethanol extract of Sophora japonica (SJEE) by the broth dilution method, and were carried out in triplicate. The antibacterial activities were examined after incubation at 37 °C for 18 h (facultative anaerobic bacteria), for 24 h (microaerophilic bacteria), and for 1-2 days (obligate anaerobic bacteria) under anaerobic conditions, used a mix of H2 and nitrogen (N2) (5/95%) or N2/carbon dioxide (CO2)/H2 (85/10/5%) to remove oxygen. MICs were determined as the lowest concentration of test samples that resulted in a complete inhibition of visible growth in the broth. MIC50s, defined as MICs at which, 50% of MIC of oral bacteria were inhibited, were determined. Following anaerobic incubation of MICs plates, the minimum bactericidal concentrations (MBCs) were determined on the basis of the lowest concentration of SJEE that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. Ampicillin, gentamicin, erythromycin, and vancomycin (Sigma) were used as standard antibiotics in order to compare the sensitivity of SJEE against oral bacteria.
Checkerboard dilution test 
The antibacterial effects of a combination of SJEE and antibiotics were assessed by the checkerboard test as previously described [23,24]. The antimicrobial combinations assayed included SJEE with antibiotics, ampicillin, gentamicin, erythromycin, and vancomycin. Serial dilutions of two different antimicrobial agents were mixed in cation-supplemented Mueller-Hinton broth. After 24-48 h of incubation at 37 °C, the MICs were determined to be the minimal concentration at which there was no visible growth and MBCs were determined on the basis of the lowest concentration of SJEE that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. The Fractional Inhibitory Concentration (FIC)/ Fractional Bactericidal Concentration (FBC) index was calculated according to the equation:

The FIC and FBC index are the sum of the FICs and FBCs of each of the drugs, which in turn is defined as the MIC and MBC of each drug when it is used in combination divided by the MIC and MBC of the drug when it is used alone. The interaction was defined as synergistic if the FIC and FBC index was less than or equal to 0.5, additive if the FIC and FBC index was greater than 0.5 and less than or equal 1.0, indifferent if the FIC and FBC index was greater than 1.0 and less than or equal to 2.0, and antagonistic if the FIC and FBC index was greater than 2.0 [23].
Biofilm formation assay
Evaluation of the effect of SJEE on biofilm formation of oral bacteria by crystal violet biofilm formation assay was performed according to previous studies [25,26]. Briefly, 200 μL aliquots of treated oral bacteria (final concentration of 1.0×106 CFU/mL) at sub-lethal dose of SJEE (1/2 MIC) plus antibiotics (1/8 MIC) was transferred to a flat-bottomed sterile polystyrene micro plate, and incubated for 24-48 h at 37 °C under anaerobic conditions to form biofilm. Then, cells were washed with Phosphate-Buffered Saline (PBS), stained with 0.1% (wt/vol) crystal violet solution for 15 min, washed with PBS, and de-stained with 96% ethanol 10 min in order to fix the cells. Thereafter, the wells were rinsed and air-dried. 33% (vol/ vol) acetic acid was then added to each well and biofilm formation was quantified by measuring the absorbance of the solution at 540 nm using a micro plate reader (BMG LABTECH, USA).
Time-kill and growth inhibition curves assay
Bactericidal activities of SJEE and antibiotics under study were also evaluated using time kill curves on oral bacteria. Tubes containing Mueller-Hinton supplemented to which antibiotics had been added at concentrations of the 1/2 MIC were inoculated with a suspension of the test strain, giving a final bacterial count between 5~7×106 CFU/mL. The tubes were thereafter incubated at 37 °C in an anaerobic chamber and viable counts were performed at 0, 0.5,1, 2, 3, 4, 5, 6, 12 and 24 h after addition of antimicrobial agents, on agar plates incubated for up to 48 h in anaerobic chamber at 37 °C. Antibiotic carryover was minimized by washings by centrifugation and serial 10-fold dilution in sterile phosphate-buffered saline, pH 7.3. Colony counts were performed in duplicate, and means were taken. The solid media used for colony counts were BHI agar for streptococci and BHI agar containing hemin and menadione for P. intermedia and P. gingivalis.
Statistical analysis
Experiments were performed three times and statistical analyses were performed with parametric tests (two-way analysis of variance [ANOVA] and Tukey’s test) using commercial software (SPSS 22.0). The results were expressed as mean values±standard deviations (mean±SD) and were considered significant at the level of p<0.05 or/ and <0.01.
Results and Discussion
Minimum inhibitory concentrations/minimum bactericidal concentrations of SJEE and antibiotics
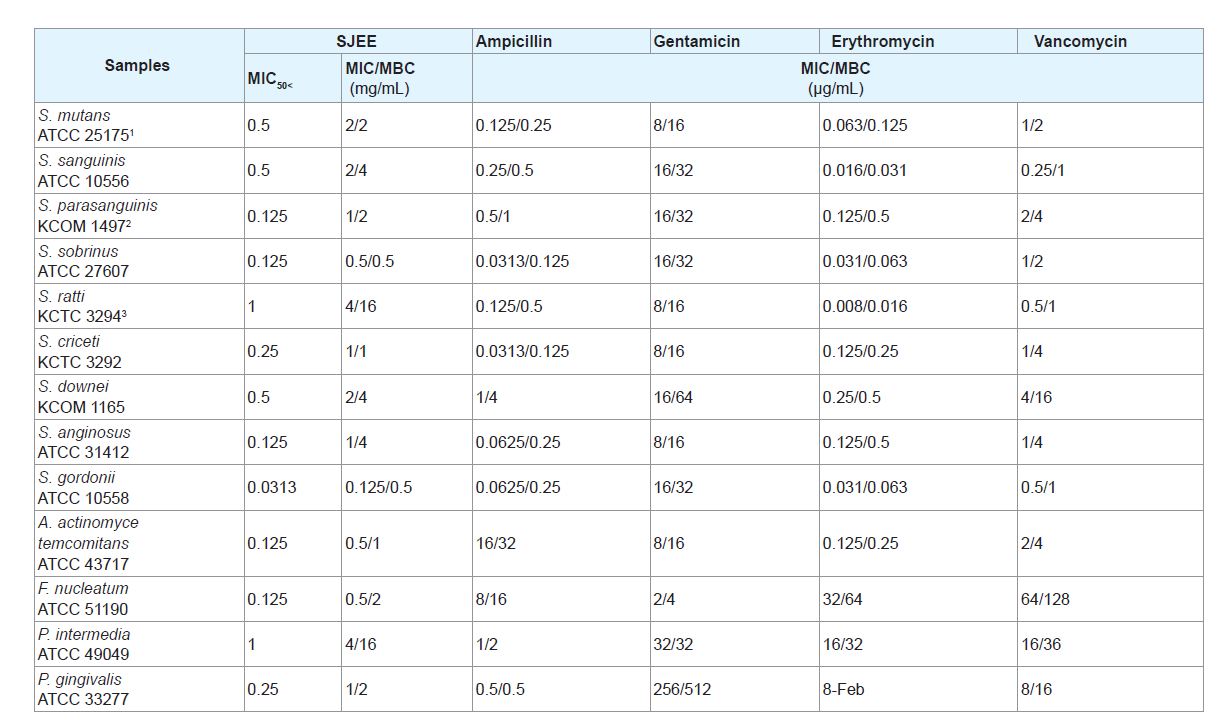
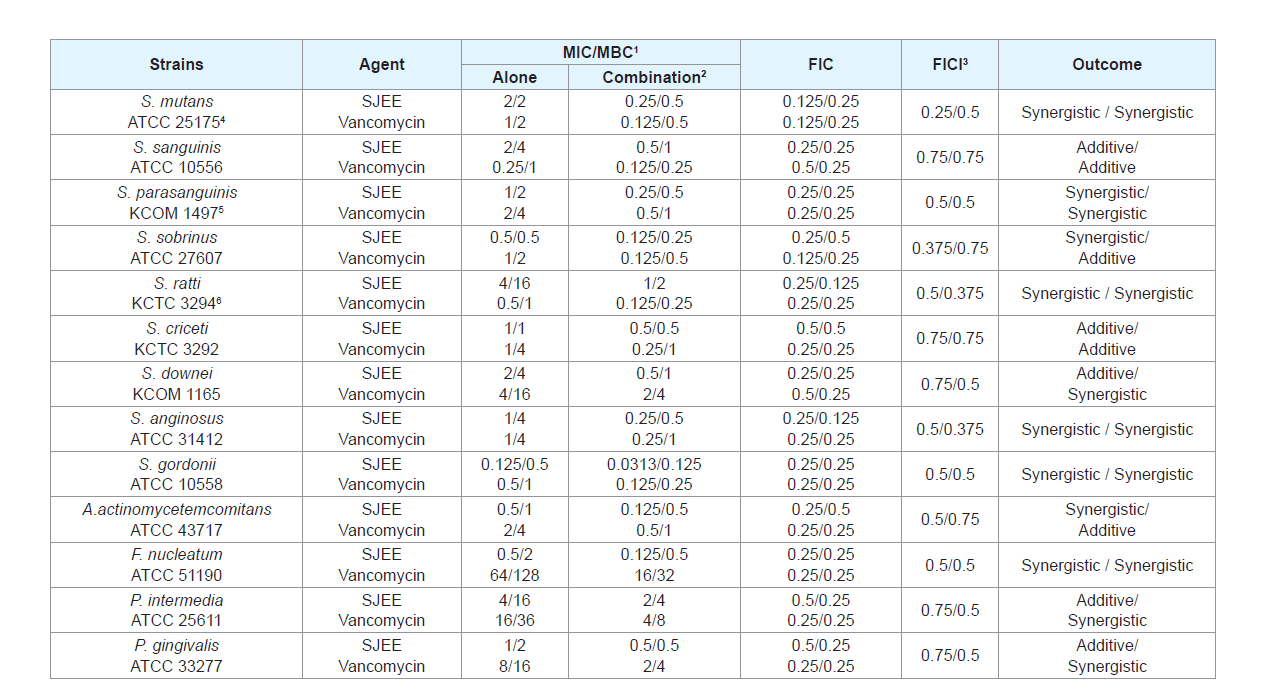
The use of natural products and herbal medicines has beendocumented in the past. They have been reported to be effective in themanagement of many infections in general. Some of these have beenassessed in the recent past for their antimicrobial potential against oralbacteria [27-29]. SJEE was evaluated for their antimicrobial activities against thirteen oral bacterial species present in the oral cavity. The results of the antimicrobial activity showed that SJEE exhibited antimicrobial activities against cariogenic bacteria at MICs, 0.125 to 4 mg/mL; MBCs, 0.5 to 16 mg/mL, against periodontopathogenic bacteria at MICs, 0.5 to 4 mg/mL; MBCs, 1 to 16 mg/mL and for ampicillin, either MIC/MBCs 0.0625/1 or 1/4 μg/mL; for gentamicin, either MIC/MBCs 2/8 or 256/512 μg/mL; for erythromycin, either 0.016/0.031 or 32/64 μg/mL; for vancomycin, either 0.25/1 or 64/128 μg/mL on tested all bacteria (Table 1). The MIC50 and MIC90 ranges of SJEE were from 0.313 to 1 mg/mL and 0.125 to 4 mg/mL, respectively. The SJFF showed stronger antimicrobial activity against S. sobrinus, S. gordonii, A. actinomycetemcomitans and F. nucleatum at MIC/MBC, 0.125/0.5-0.5/2 mg/mL than another bacteria at MIC/ MBC, 1-4/1-16 mg/mL.
Table 1: Antibacterial activity of 50% ethanol extract of Sophora japonica (SJEE) and antibiotics in oral bacteria.
Synergistic effect of SJEE with antibiotics
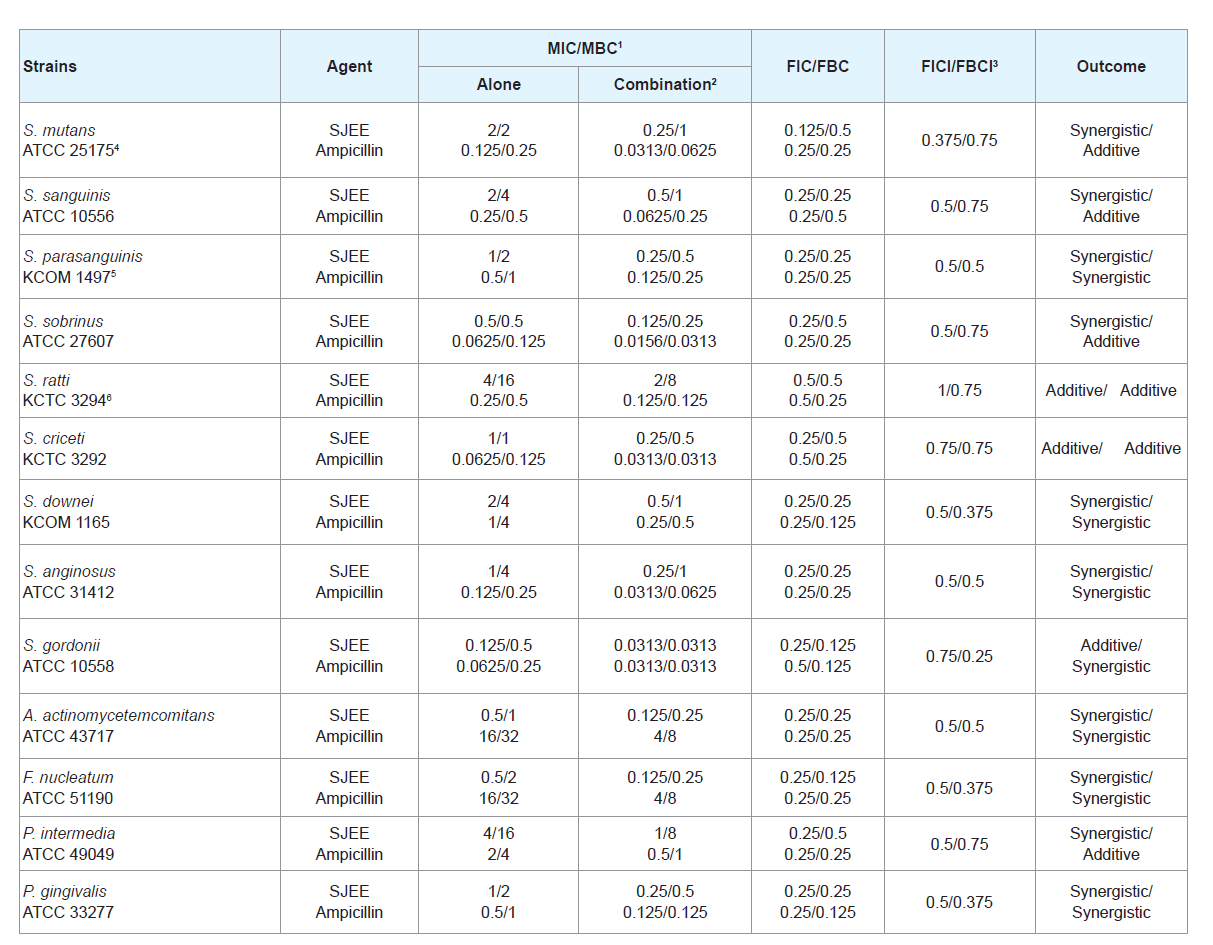
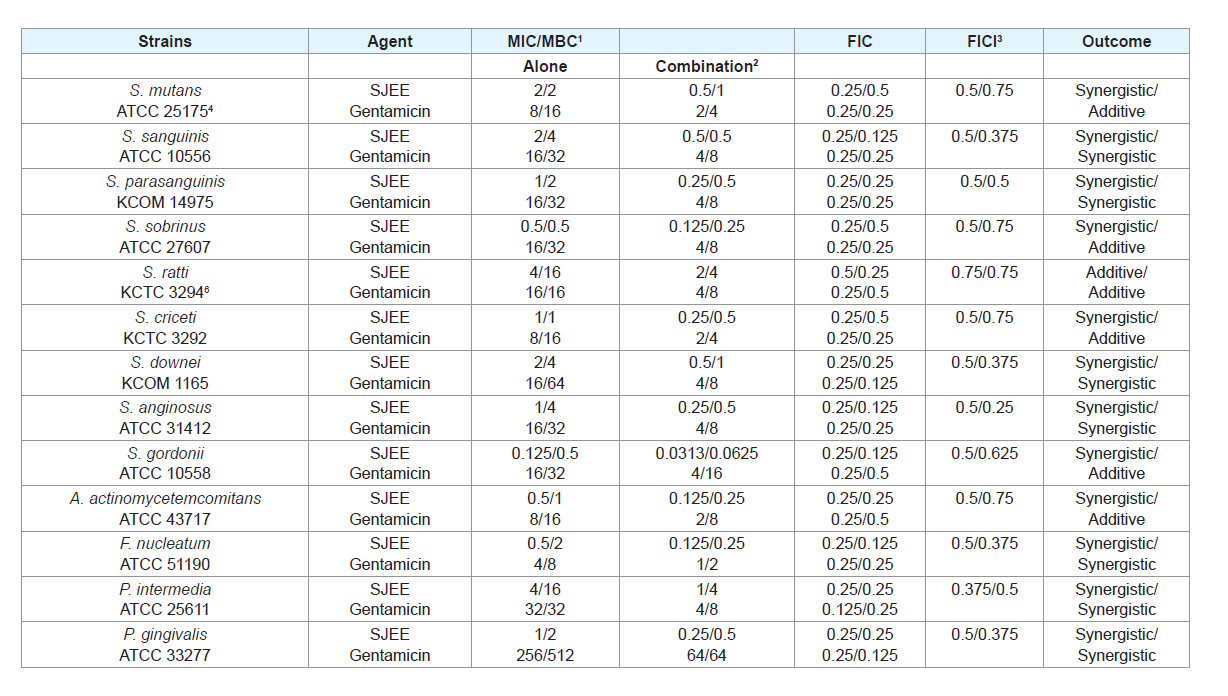
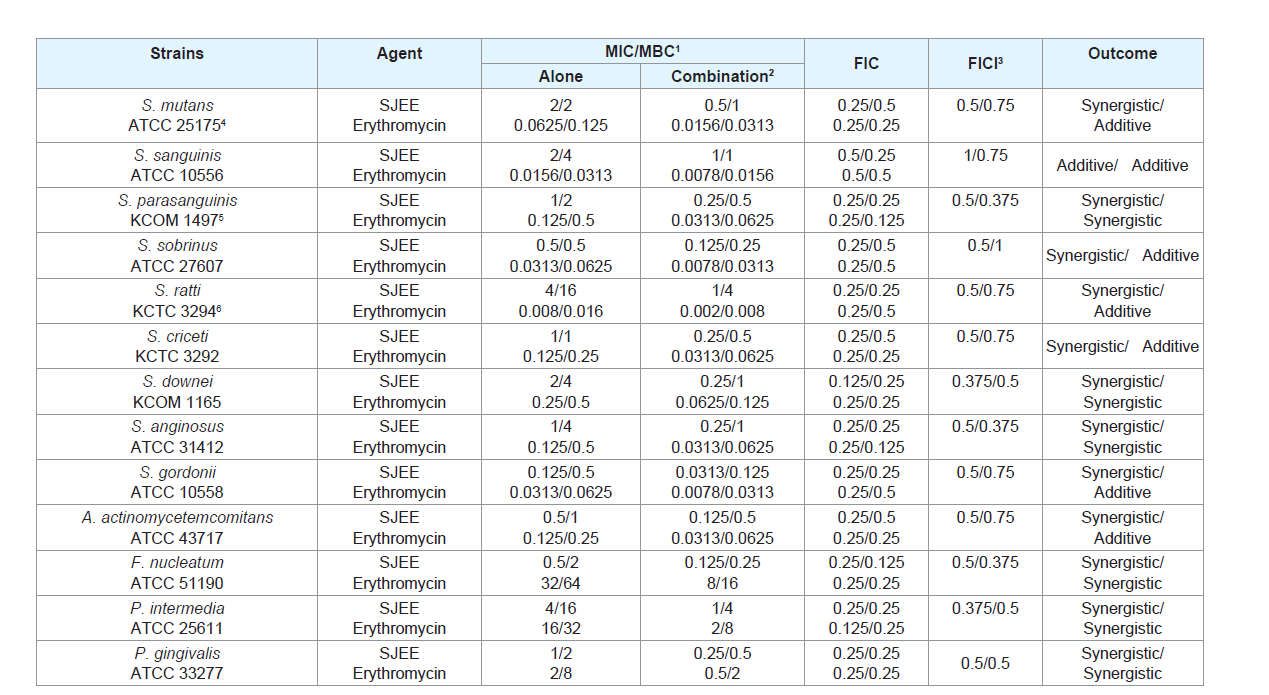
Many antimicrobial preparations, such as conventional antibiotics, chlorhexidine (CHX), phenolic compounds and triclosan, can inhibit bacteria biofilm effectively [30,31]. However, extensive use of these antimicrobial agents can lead to some sideeffects, such as tooth staining, calculus formation, drug resistance and gastrointestinal reactions [32,33]. Therefore, searching for new antimicrobial molecules, which exhibit few or no side effects and long term retention in oral cavity, has been intensified in recent years [34]. The synergistic effects of SJEE with antibiotics or with antibiotics were evaluated in oral bacteria (Tables 2-5). In combination with ampicillin, SJEE was reduced ≥4-fold in tested bacteria, except S. ratti, S. criceti, and S. gordnii, producing a synergistic effect as defined by FICI≤0.5. The MBC for ampicillin was shown synergistic effects in S. parasanguinis, S. downei, S. anginosus, S. gordonii, A. actinomycetemcomitans, F. nucleatum, and P. gingivalis by FBCI≤0.5 (Table 2). In combination with SJEE, the MIC for gentamicin was reduced ≥4-8-fold in all tested bacteria, expect S. ratti by FICI≤0.5 and MBC in S. mutans, S. sobrinus, S. ratti, S. criceti, S. gordonii, and A. actinomycetemcomitans by FBCI≤0.5 (Table 3). Moreover, the MIC for erythromycin with SJEE was reduced ≥4-fold in tested bacteria, except S. sanguinis, producing a synergistic effect as defined by FICI≤0.5 and the MBC for erythromycin was shown synergistic effects, except S. mutans, S. sanguinis, S. sobrinus, S. rattii, S. criceti, S. gordonii, and A. actinomycetemcomitans by FBCI≥0.75 (Table 4). In combination with SJEE, the MIC for vancomycin was reduced ≥4 fold in tested all bacteria, except S. sanguinis, S. criceti, S. downei, P.intermedia, and P. gingivalis, producing a synergistic effect as defined by FICI≤0.5. The MBC for vancomycin was shown synergistic effects in S. sanguinis, S. sobrinus, S. criceti, A. actinomycetemcomitans, and P. gingivalis by FBCI≤0.75 (Table 5).
Table 2: Synergistic effects of 50% ethanol extract of Sophora japonica (SJEE) with ampicillin against oral bacteria.
Table 3: Synergistic effects of 50% ethanol extract of Sophora japonica (SJEE) with gentamicin against oral bacteria.
Table 4: Synergistic effects of 50% ethanol extract of Sophora japonica (SJEE) with erythromycin against oral bacteria.
Table 5: Synergistic effects of 50% ethanol extract of Sophora japonica (SJEE) with vancomycin against oral bacteria.
Anti-biofilm formation of SJEE with antibiotics
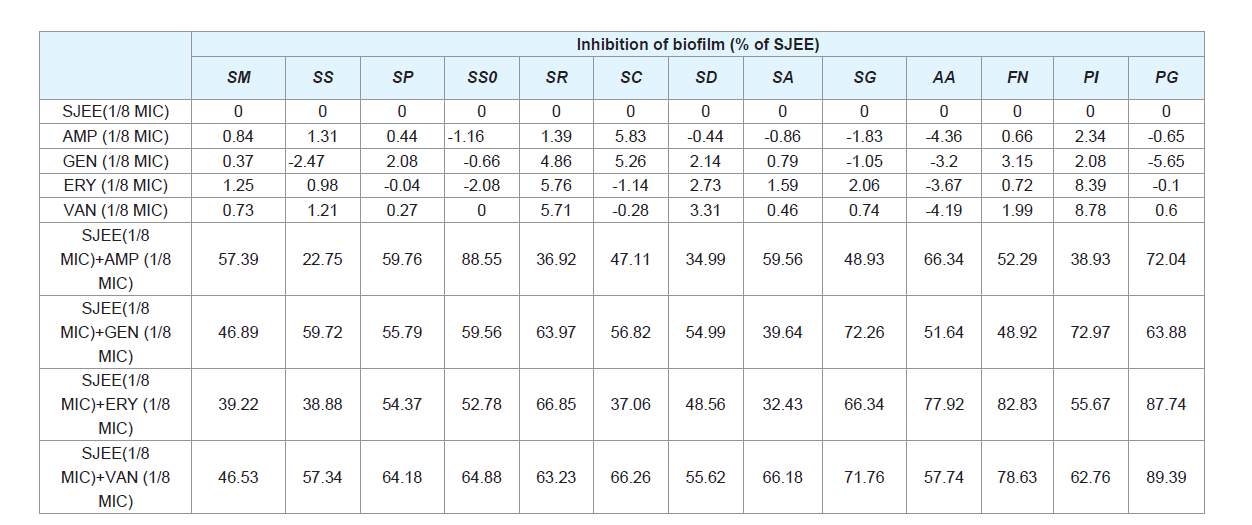
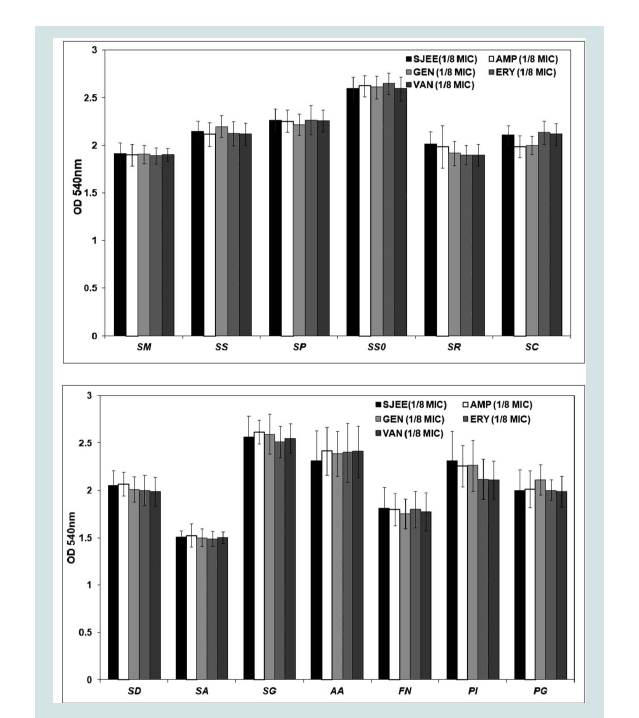
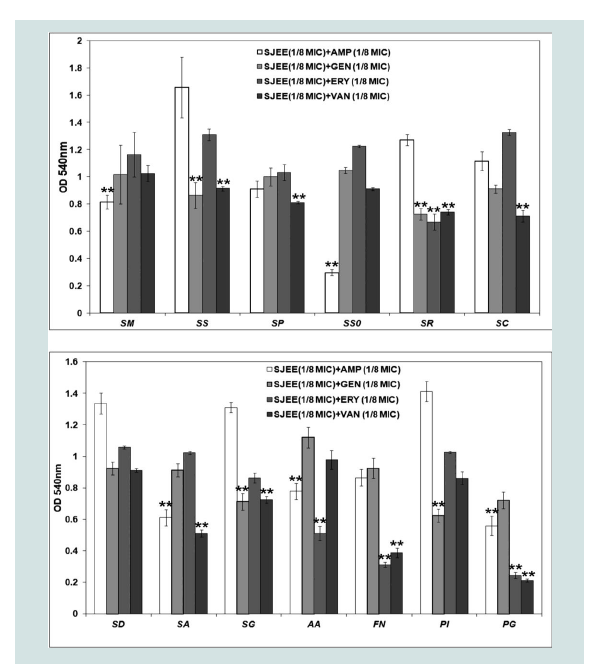
Oral diseases, such as dental caries, periodontal disease are directly linked with the ability of bacteria to form biofilm [3,4,7,9]. Prenylated flavonoids are widely distributed in the plant world and prenylation elevates hydrophobicity on the basic structure of the molecule, enhancing flavonoid biological functions [35]. Prenylation facilitates flavonoid interaction with biofilm and uptake into bacteria through the membrane, enhancing inhibitory effects of the flavonoids on P. gingivalis growth and biofilm formation [36,37]. The isorhamnetin-3-O-β-D-ru-tinoside from the dried flowers of S. japonica, which significantly inhibited the action of sortase A (SrtA) from S. mutans [15,40]. The maltol-3-O-(4’-O-cis-p-coumaroyl- 6’-O-(3- hydroxy-3-methylglutaroyl))-β-glucopyranoside from the dried flowers of S. japonica showed the strongest inhibitory effect on saliva-induced aggregation in S. mutans with an IC50 value of 58.6 μM [38]. A gradual increase of biomass for oral bacteria was noted up to 24 h in both SJEE and antibiotics ((Table 6) and (Figure 1)). S. sobrinus was the most susceptible to SJEE (1/8 MIC) plus AMP (1/8 MIC) with 88.1% inhibition as compared to SJEE (1/2 MIC) at the end of 24 h of incubation. S. gordonii indicated the most inhibition to SJEE (1/8 MIC) plus GEN (1/8 MIC) with 76.12%. SJEE (1/8 MIC) plus ERY (1/8 MIC) and SJEE (1/8 MIC) plus VAN (1/8 MIC) were shown with 89.42% and 94.03% inhibition for P. gingivalis biofilm, respectively.
Time kill of SJEE with antibiotics
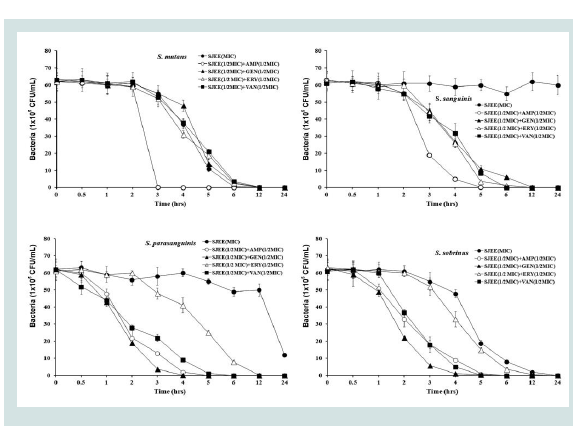
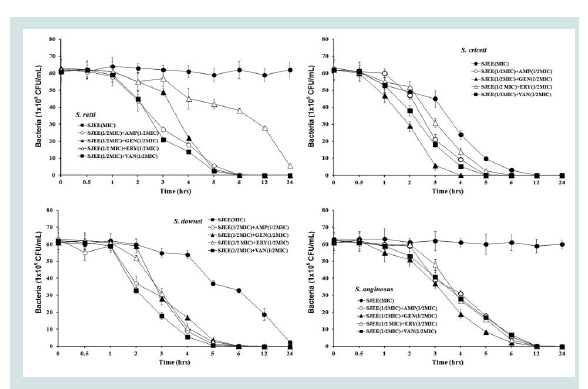
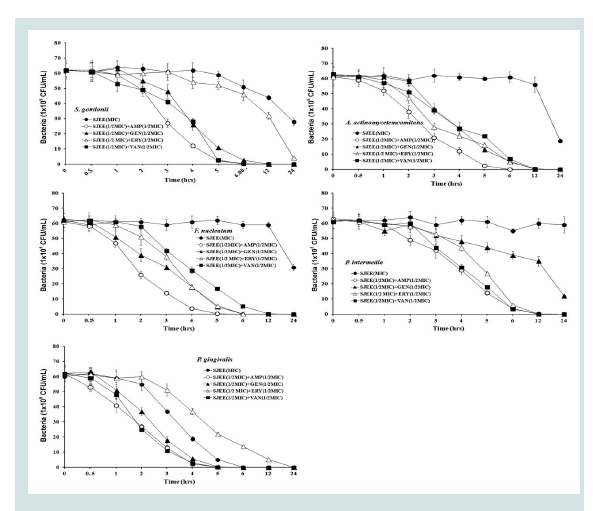
Previous studies had reported the antimicrobial activities and mechanisms of SJEE against several kinds of bacteria [21,22].However, the type of microorganisms and their cell membrane structure and composition could play an important role in the susceptibility to antimicrobials [39,40]. The MIC value was similar to the results that MIC values of SJEE against Staphylococcus aureus and Aeromonas hydrophila were 20 μg/mL and 50 μg/mL, respectively [22,41,42]. The bacterial effect of SJEE with antibiotics, ampicillin, gentamicin, erythromycin, and vancomycin against oral bacteria was confirmed by time kill curve experiments. The SJEE (MIC or 1/2 MIC) alone resulted rate of killing increasing or not changing in CFU/ml at time defpendent manner, with a more rapid rate of killing by SJEE (1/2 MIC) with ampicillin, gentamicin, erythromycin, or/and vancomycin (1/2 MIC) (Figures 2-4). A strong bactericidal effect was exerted in drug combinations.
Conclusion
In conclusion, these findings suggest that crude extract of S.japonica exhibited a wide range of pharmacological effects establish the conditions required of a novel cariogenic bacteria and periodontal pathogens, particularly bacteroides species drug and may be useful in the future in the treatment of oral bacteria biofilm.
Figure 1: Anti-biofilm effect of different concentrations of SJEE alone or/ and antibiotics on biofilm formation of oral bacteria, SM, SS, SP, SSO, SR, and SC. Cells stained with 0.1% (wt/vol) crystal violet solution for 15 min, washed with PBS, and de-stained with 96% ethanol 10 min in order to fix the cells. Thereafter, acetic acid was then added to each well and biofilm formation was quantified by measuring the absorbance of the solution at 540 nm using a micro plate reader. Data points are the mean values±S.E.M. of six experiments.
SM: Streptococcus mutans; SS: Streptococcus sanguinis; SP:Streptococcus parasanguinis; SSO: Streptococcus sobrinus; SR:Streptococcus ratti; SC: Streptococcus criceti.
SM: Streptococcus mutans; SS: Streptococcus sanguinis; SP:Streptococcus parasanguinis; SSO: Streptococcus sobrinus; SR:Streptococcus ratti; SC: Streptococcus criceti.
Figure 2: Anti-biofilm effect of different concentrations of SJEE alone or/andantibiotics on biofilm formation of oral bacteria, SD, SA, SG, AA, FN, PI, andPG. Cells stained with 0.1% (wt/vol) crystal violet solution for 15 min, washed with PBS, and de-stained with 96% ethanol 10 min in order to fix the cells. Thereafter, acetic acid was then added to each well and biofilm formation was quantified by measuring the absorbance of the solution at 540 nm using a microplate reader. Data points are the mean values±S.E.M.
**Significantly different from the control group (1/8 MIC SJEE or antibiotics) (P<0.01) of six experiments.
SD: Sreptococcus downei; SA: Streptococcus anginosus; SG:Streptococcus gordonii; AA: Aggregatibacter actinomycetemcomitans; FN:Fusobacterium nucleatum; PI: Prevotella intermedia; PG: Porphylomonasgingivalis
**Significantly different from the control group (1/8 MIC SJEE or antibiotics) (P<0.01) of six experiments.
SD: Sreptococcus downei; SA: Streptococcus anginosus; SG:Streptococcus gordonii; AA: Aggregatibacter actinomycetemcomitans; FN:Fusobacterium nucleatum; PI: Prevotella intermedia; PG: Porphylomonasgingivalis
Figure 3: Time-kill curves of MIC of SJEE alone and its combination with 1/2 MIC of AMP, GEN, ERY, or/and VAN against S. mutans, S. sanguinis, S. parasanguinis, and S. sobrinus. Bacteria were incubated with SJEE (●),SJEE+AMP (○), SJEE+GEN (▲), SJEE+ERY (△), and SJEE+VAN (■) over time. Data points are the mean values±S.E.M. of six experiments. CFU, colony forming units.
Figure 4: Time-kill curves of MIC of SJEE alone and its combination with 1/2 MIC of AMP, GEN, ERY, or/and VAN against S. ratti, S. criceti, S. downei, and S. anginosus. Bacteria were incubated with SJEE (●), SJEE+AMP (○), SJEE+GEN (▲), SJEE+ERY (△), and SJEE+VAN (■) over time. Data points are the mean values±S.E.M. of six experiments. CFU, colony forming units.
Figure 5: Time-kill curves of MIC of SJEE alone and its combination with 1/2 MIC of AMP, GEN, ERY, or/and VAN against S. gordonii, A.actinomycetemcomitans, F. nucleatum,P. intermedia, and P. gingivalis.Bacteria were incubated with SJEE (●), SJEE+AMP (○), SJEE+GEN (▲),SJEE+ERY (△), and SJEE+VAN (■) over time. Data points are the meanvalues±S.E.M. of six experiments. CFU, colony forming units.
References
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, et al. (2010) The human oral microbiome. J Bacteriol 192: 5002-5017.
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721-5732.
- Do T, Devine DA, Marsh PD (2013) Oral biofilms: molecular analysis, challenges, and future prospects in dental diagnostics. Clin Cosmet Investig Dent 5: 11-19.
- Larsen T, Fiehn NE (2017) Dental biofilm infections-an update. APMIS 125: 376-384.
- Grossner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, et al. (2006) Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol 33: 478-484.
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, et al. (2017) Dental caries. Nat Rev Dis Primers 3: 17030.
- de Soet JJ (2015) Dissertation 25 years after date 42. Dental caries and the role of specific bacteria. Ned Tijdschr Tandheelkd 122: 525-531.
- Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, et al. (2013) Correlation of mast cells in periodontal diseases. J Indian Soc Periodontol 17: 63-67.
- Ardila CM, López MA, Guzmán IC (2011) Positive correlations between presence of gram negative enteric rods and Porphyromonas gingivalis in subgingival plaque. Acta Odontol Latinoam 24: 15-19.
- Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3: 17038.
- Nazir MA (2017) Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 1: 72-80.
- Garton BJ, Ford PJ (2012) Root caries and diabetes: risk assessing to improve oral the systemic health outcomes. Aust Dent J 57: 114-122.
- Ramakrishna Y, Goda H, Baliga MS, Munshi AK (2011) Decreasing cariogenic bacteria with a natural, alternative prevention therapy utilizing phytochemistry (plant extracts). J Clin Pediatr Dent 36: 55-63.
- Ishida H, Umino T, Tsuji K, Kosuge T (1989) Studies on the antihemostatic substances in herbs classified as hemostatics in traditional Chinese medicine. I. on the antihemostatic principles in Sophora japonica L. Chem Pharm Bull 37: 1616-1618.
- He X, Bai Y, Zhao Z, Wang X, Fang J, et al. (2016) Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: a review. J Ethnopharmacol 187: 160-182.
- Man KM, Chen WC, Wang HM, Chen HY, Shen JL, et al. (2012) A randomized, double-blind, placebo-controlled trial of a Chinese herbal Sophora flower formula in patients with symptomatic haemorrhoids: a preliminary study. Afr J Tradit Complement Altern Med 10: 343-351.
- Tang Y, Lou F, Wang J, Zhuang S (2001) Four new isoflavone triglycosides from Sophora japonica. J Nat Prod 64: 1107-1110.
- Gorbacheva LA, Grishkovets VI, Drozd GA, Chirva VYa (1996) Triterpene glycosides from Sophora japonica L. seeds. Adv Exp Med Biol 404: 501-504.
- Sun A, Sun Q, Liu R (2007) Preparative isolation and purification of flavone compounds from Sophora japonica L. by high-speed counter-current chromatography combined with macroporous resin column separation. J Sep Sci 30: 1013-1018.
- Abdallah HM, Al-Abd AM, Asaad GF, Abdel-Naim AB, El-halawany AM (2014) Isolation of antiosteoporotic compounds from seeds of Sophora japonica. PLoS One 9: e98559.
- Kimura M, Yamada H (1984) Interaction in the antibacterial activity of flavonoids from Sophora japonica L. to Propionibacterium. Yakugaku Zasshi 104: 340-346.
- Danilevskiĭ NF, Antonishin BV (1982) Antimicrobial activity of a tincture of Japanese pagoda tree (Sophora japonica) and of the essential oil of sweet flag (Acorus calamus). Mikrobiol Zh 44: 80-82.
- Cha JD, Jeong MR, Jeong SI, Lee KY (2007) Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens. J Microbiol Biotechnol 17: 858-864.
- Ma XH, Zheng CJ, Han LY, Xie B, Jia J, et al. (2009) Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov Today 14: 579-588.
- Jeong SI, Kim BS, Keum KS, Lee KH, Kang SY, et al. (2013) Kaurenoic acid from Aralia continentalis inhibits biofilm formation of Streptococcus mutans. Evid Based Complement Alternat Med 2013: 160592.
- O’Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp.
- Khalid M, Hassani D, Bilal M, Butt ZA, Hamayun M, et al. (2017) Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia Arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch Oral Biol 81: 175-185.
- Choi HA, Cheong DE, Lim HD, Kim WH, Ham MH, et al. (2017) Antimicrobial and anti-biofilm activities of the methanol extracts of medicinal plants against dental pathogens Streptococcus mutans and Candida albicans. J Microbiol Biotechnol 27: 1242-1248.
- Ferrazzano GF, Roberto L, Catania MR, Chiaviello A, De Natale A, et al. (2013) Screening and scoring of antimicrobial and biological activities of Italian vulerary plants against major oral pathogenic bacteria. Evid Based Complement Alternat Med 2013: 316280.
- Pandit S, Kim HJ, Park SH, Jeon JG (2012) Enhancement of fluoride activity against Streptococcus mutans biofilms by a substance separated from Polygonum cuspidatum. Biofouling 28: 279-287.
- Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F (2012) Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 39: 1042-1055.
- Baehni PC, Takeuchi Y (2003) Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis 9 (Suppl 1): 23-29.
- Bhardwaj P, Krishnappa S (2014) Various approaches for prevention of dental caries with emphasis on probiotics: a review. IOSR J Dent Med Sci 13: 62-67.
- Wang HY, Cheng JW, Yu HY, Lin L, Chih YH, et al. (2015) Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta Biomater 25: 150-161.
- Ming LG, Chen KM, Xian CJ (2013) Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol 228: 513-521.
- Kariu T, Nakao R, Ikeda T, Nakashima K, Potempa J, et al. (2017) Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. J Periodontal Res 52: 89-96.
- Shahzad M, Millhouse E, Culshaw S, Edwards CA, Ramage G, et al. (2015) Selected dietary (poly) phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct 6: 719-729.
- Yang WY, Won TH, Ahn CH, Lee SH, Yang HC, et al. (2015) Streptococcus mutans sortase A inhibitory metabolites from the flowers of Sophora japonica. Bioorg Med Chem Lett 25: 1394-1397.
- Nowotarska SW, Nowotarski KJ, Friedman M, Situ C (2014) Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 19: 7497-7515.
- Roces C, Rodríguez A, Martínez B (2012) Cell wall-active bacteriocins and their applications beyond antibiotic activity. Probiotics Antimicrob Proteins 4: 259-272.
- Park SJ, Shin EH, Hahm TS (2009) Biological activities in the extract of flos Sophora japonica L. J Korean Soc Food Sci Nutr 38: 9-13.
- Chen Y, Zhang YZ, Sun SL, Yao WR (2008) Chemical composition and antimicrobial activity of essential oil from Flos Sophorae Immaturus. Mod Food Sci Technol 24: 318-321.