Journal of Food Processing & Beverages
Download PDF
Review Article
*Address for Correspondence : H. P. Vasantha Rupasinghe, Department of Environmental Sciences, Faculty of Agriculture, Dalhousie University, Room 219-3 Cox Building, 50 Pictou Road (PO Box 550), Truro, NS B2N 5E3 Canada, Tel: 902-893-6623; Fax: 902-893-1404; E-mail: vrupasinghe@dal.ca
Citation: Ruchira Nandasiri, HP Vasantha Rupasinghe. Inhibition of Low Density Lipoprotein Oxidation and Angiotensin Converting Enzyme in vitro by Functional Fruit Vinegar Beverages. J Food Processing & Beverages. 2013;1(1): 5.
Copyright © 2013 Vasantha Rupasinghe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 1
Submission: 21 August 2013 | Accepted: 19 September 2013 | Published: 27 September 2013
The final three levels of acetic acid concentration (5, 10 and 15 mg acetic acid equivalents/g) was obtained by blending the fermented beverage with the respective fruit juices (apple, blueberry, cranberry or tomato) until they reached the final three levels of acetic acid concentrations (5, 10 and 15 mg acetic acid equivalents/g). After blending, vinegar beverage samples were again filtered through four layers of cheesecloth to remove sediments [8]. Filtered fruit vinegar samples were then pasteurized using a batch type pasteurizer (Model SK-620X-BLT, Advantage, Greenwood, IN, USA) at 90°C for 5 minutes. Soon after pasteurization, hot samples were bottled into sterilized containers and stored at -20°C until further use.
LDL oxidation inhibitionAll four fruit vinegar beverages at three acetic acid concentrations (5, 10, and 15 mg acetic acid equivalents/g) were incubated with LDL reaction mixture induced with peroxyl radical generator (AAPH) to determine the level of LDL oxidation inhibition in vitro. Results indicated that blueberry and cranberry vinegar beverages have higher LDL oxidation inhibition capacities than apple and tomato vinegar beverages in vitro (Table 2). Furthermore, all the vinegar beverages at 5 and 15 mg acetic acid equivalents/g concentration demonstrated over 50% LDL oxidation inhibition in vitro suggesting potential beneficial antioxidant properties of fruit vinegar beverages. However, the interesting finding of the current study was that both blueberry (~ 80%) and cranberry (~ 60%) showed similar percentages of inhibitions in vitro at all three levels of acidity. Thus, apple and tomato exhibited acetic acid concentration dependent relationships for the antioxidant capacities (higher the level of fruit juice, higher the antioxidant properties).
ACE InhibitionAll fruit vinegar beverages (above 10 mg acetic acid equivalents/g) exhibited a concentration dependent enzyme inhibition for ACE assay in vitro (Table 2). When the concentration of the acetic acid increased, the level of inhibition also increased (from 10 to 15 mg acetic acid equivalents/g). Tomato vinegar beverage exhibited the lowest inhibition in vitro (16-29%) whereas cranberry vinegar beverage exhibited the highest (54-92%). Furthermore, both blueberry and apple at 15 mg acetic acid equivalents/g concentration also demonstrated over 50% inhibition of ACE in vitro. Results of the current study are in agreement with the potential antihypertensive properties of fruit vinegar beverages in vitro.
Inhibition of Low Density Lipoprotein Oxidation and Angiotensin Converting Enzyme in vitro by Functional Fruit Vinegar Beverages
Ruchira Nandasiri and H. P. Vasantha Rupasinghe*
- Department of Environmental Science, Faculty of Agriculture, Dalhousie University, P.O. Box 550, Truro, Nova Scotia, Canada B2N 5E3 117543
*Address for Correspondence : H. P. Vasantha Rupasinghe, Department of Environmental Sciences, Faculty of Agriculture, Dalhousie University, Room 219-3 Cox Building, 50 Pictou Road (PO Box 550), Truro, NS B2N 5E3 Canada, Tel: 902-893-6623; Fax: 902-893-1404; E-mail: vrupasinghe@dal.ca
Citation: Ruchira Nandasiri, HP Vasantha Rupasinghe. Inhibition of Low Density Lipoprotein Oxidation and Angiotensin Converting Enzyme in vitro by Functional Fruit Vinegar Beverages. J Food Processing & Beverages. 2013;1(1): 5.
Copyright © 2013 Vasantha Rupasinghe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 1
Submission: 21 August 2013 | Accepted: 19 September 2013 | Published: 27 September 2013
Abstract
Fruit vinegar beverages have been becoming popular as a part of the healthy diet. The current study investigated the antioxidant and invitro low density lipoprotein (LDL) oxidation inhibition and angiotensin converting enzyme (ACE) properties of four different fruit vinegar beverages: apple, blueberry, cranberry and tomato at 5, 10, and 15 mg acetic acid equivalents/g concentrations. Blueberry vinegar beverage showed the highest antioxidant capacity regardless the acetic acid concentration. Over 50% LDL oxidation inhibition in vitro was observed for cranberry (mean ± SD) (68 ± 1.7%) and blueberry (85 ± 1.5%) vinegar beverages at the three acetic acid concentrations. Both cranberry (92 ± 0.2%) and blueberry (60 ± 0.7%) vinegar beverages had higher levels of ACE inhibition in vitro at 15 mg acetic acid equivalents/g concentration. Comparatively, tomato vinegar beverage showed lower antioxidant and ACE inhibition properties in vitro. Thus, findings of the current study suggest that functional beverages, such as fruit vinegar beverages, could be an alternative to drugs in controlling hypertension and hypercholesterolemia upon the confirmation of the results, using an animal model in vivo.Keywords
Antioxidants; ACE inhibition; LDL oxidation; Acetic acid; Hypertension; Fruit vinegar beveragesIntroduction
Fruits and vegetables are known to be a good source of biologically active plant secondary metabolites such as polyphenol compounds [1]. The complex mixture of phytochemicals present in fruits and vegetables provides protective health benefits, mainly through additive and/or synergistic effects [2]. Furthermore, these polyphenol compounds have been found to exhibit strong antioxidant properties both in vitro and in vivo [1]. Even though many studies have investigated the in vitro antioxidant properties including the effects of apples [2], berries [1,3] and tomatoes [4] on inhibition of LDL oxidation, there are a very few studies conducted to investigate the invitro antioxidant properties of functional fruit vinegar beverages to our best understanding.Fruit vinegar beverages are one of the emerging functional beverages in the North American market. A fruit vinegar beverage is defined as a beverage that has been fermented from at least one kind of fruit and each litre of beverage must contain more than 300 g of fruit juice [5]. Fruit vinegar beverages have been categorized into two different types based on their acetic acid concentration: fruit vinegar beverage, which is low in acetic acid (< 3% v/v) and concentrated fruit vinegar beverage, which is high in acetic acid (5 - 7% v/v) [5]. Total sugar content, the titratable acidity level, total soluble solids content and density of the fruit vinegar beverage may depend on the method of fermentation and the concentration of acetic acid content [5,6]. The key organic acid associated with fruit vinegar beverage is acetic acid [5,6]. Furthermore, these fruit vinegars consist of high concentrations of polyphenolic compounds [7]. However, to produce a consumer acceptable fruit vinegar beverage, the choice of raw materials and the method of acetification are major important factors to be considered [8].
Apples, berries and tomatoes have distinct polyphenolic compositions and are well known for their health promoting properties [9-11]. Although, there are innumerable studies on the health benefits of fresh as well as processed fruit products, effects of fruit vinegar beverages on vascular function and blood pressure are largely unknown. Therefore, this study was carried out as an initial step to assess the in vitro antioxidant capacities, LDL oxidation inhibition and ACE inhibition ability of a fruit vinegar beverage developed using apples, berries and tomatoes. The current study evaluated the antioxidant and antihypertensive properties of four different fruit vinegar beverages: apple, blueberry, cranberry and tomato at 0.5%, 1.0%, and 1.5% acetic acid concentrations.
Materials and Methods
Samples and Chemical Reagents
Apples and tomatoes were purchased from a local grocery store (Sobeys, Truro, NS, Canada), Concentrated cranberry juice was purchased from a commercial cranberry juice manufacturer (Cranberry Acres, Berwick, NS, Canada) and blueberry juice (100% juice) was purchased from a commercial blueberry juice manufacturer (Van Dyke, Caledonia, Queens Co., NS, and Canada).
Sample Preparation
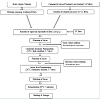
Fruit vinegar beverages were developed using the methods by Su and Chien [8] and Nakamura [12] with modifications. The method in brief is as follows (Figure 1). Good quality raw materials (apples and tomato) were selected and washed and then pressed using an X1 hydraulic plate presser (Model JVH 56C17F5323J, Marathon, WI, USA). A total of about 8 kg of apple or tomato fruits were required to obtain 4 L of juice (about 50% yield from the X1 hydraulic plate presser). The pressed juice sample was filtered using four layers of cheesecloth and then adjusted or concentrated to approximately 20° Brix (added sucrose to a final sugar concentration of around 120 g/L depending on the type of fruit) (Figure 1). In general, alcoholic fermentation is usually carried out until all the sugars were converted into ethanol. These juice samples (4 L) were then subjected to controlled alcohol fermentation in fermentation vats using yeast (Saccharomyces cerevisiae) to obtain the desired final alcohol concentration between 2% to 5% (v/v). Alcoholic fermentation required five to seven days depending on the type of fruit (24°C, in dark condition) [8]. Fermented juice was filtered again through four layers of cheesecloth to remove yeast from the fermentation system. Once the alcohol level reached up to 2% to 5% (v/v), alcoholic fermentation was stopped and acetic acid fermentation was initiated. This was carried out using the quick method where the submerged culture of bacteria (from previously produced vinegar) was used (volume ratio of 3 fermented juice: 2 acetic acid culture) and continuous oxygen supply was manipulated through aeration [8,12]. Acetic fermentation was carried out in 2 L volume glass containers in three replicates. It continued for five to six days, depending on the type of fruit (28°C, continuous aeration). The titratable acidity (%) was monitored daily until the required level of acidity (2% v/v) was obtained.
Antioxidant Properties of the Fruit Vinegar Beverages
Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP assay was performed exactly as described by Rupasinghe et al. [13]. Trolox (Sigma-Aldrich Ltd., Oakville, ON) solution was used as the standard at concentrations of 50, 100, 300, 500, 700, and 900 μmol/L. The FLUOstar OPTIMA plate reader with an incubator and an injection pump (BMG Labtech Inc., Offenburg, Germany) was programmed (BMG Labtech software) to obtain an absorption reading at 595 nm, 6 minutes after injecting the FRAP solution and shaking for 3 s. FRAP working reagent consists of 300 mmol/L acetate buffer (pH 3.6) (Sigma-Aldrich Ltd., Oakville, ON), 1 mmol/L 2,4,6-tripyridyl-s-triazine (TPTZ) solution (Sigma-Aldrich Ltd., Oakville, ON), and 20 mmol/L ferric chloride solution (Sigma-Aldrich Ltd., Oakville, ON) and was prepared fresh daily. Before the assay, the FRAP reagent as well as the samples in the microplate were warmed to 37°C. The resulting FRAP values were expressed as mmol Trolox equivalents per liter of sample.
Oxygen Radical Absorbance Capacity (ORAC) Assay
The assay was performed as mentioned in Rupasinghe et al. [13] with a few modifications. Trolox (Sigma-Aldrich Ltd., Oakville, ON) was used as the standard at concentrations of 50, 100, 300, 500, 700, and 900 μmol/L. Thirty five microliters of each sample or standard were placed in the wells of a 96-well micro-plate (COSTAR 3915, Fisher Scientific, Ottawa, ON, Canada) and 130 μL of fluorescein (K2HPO4/NaH2PO4, pH 7.0, Sigma-Aldrich Ltd., Oakville, ON) was pipetted. The plate was warmed to 37°C for 5 minutes and 35 μL of prewarmed peroxyl radical generator, 2, 2’-Azobis (2-amidinopropane) dihydrochloride (AAPH) (Sigma-Aldrich Ltd., Oakville, ON) solution was injected into the wells. The microplate was shaken for 3s after each injection of AAPH and prior to each reading. The plate was kept at 37°C throughout the experiment of approximately 50 minutes. The excitation and emission wavelengths used were 490 nm and 510 nm respectively and the fluorescence was recorded at 2 min intervals. The ORAC values were expressed as mmol Trolox equivalents per liter of sample.
Oxygen Radical Absorbance Capacity (ORAC) Assay
The assay was performed as mentioned in Rupasinghe et al. [13] with a few modifications. Trolox (Sigma-Aldrich Ltd., Oakville, ON) was used as the standard at concentrations of 50, 100, 300, 500, 700, and 900 μmol/L. Thirty five microliters of each sample or standard were placed in the wells of a 96-well micro-plate (COSTAR 3915, Fisher Scientific, Ottawa, ON, Canada) and 130 μL of fluorescein (K2HPO4/NaH2PO4, pH 7.0, Sigma-Aldrich Ltd., Oakville, ON) was pipetted. The plate was warmed to 37°C for 5 minutes and 35 μL of prewarmed peroxyl radical generator, 2, 2’-Azobis (2-amidinopropane) dihydrochloride (AAPH) (Sigma-Aldrich Ltd., Oakville, ON) solution was injected into the wells. The microplate was shaken for 3s after each injection of AAPH and prior to each reading. The plate was kept at 37°C throughout the experiment of approximately 50 minutes. The excitation and emission wavelengths used were 490 nm and 510 nm respectively and the fluorescence was recorded at 2 min intervals. The ORAC values were expressed as mmol Trolox equivalents per liter of sample.
Low Density Lipoprotein Thiobarbituric Acid Reactive Substances (LDL TBARS) Assay
LDL TBARS assay was carried out as described by Thilakarathne and Rupasinghe [14] with a few modifications. LDL isolated from human plasma (150 mmol/L NaCl, 0.01% EDTA, pH 7.4) was purchased from EMD Chemicals Inc. (Gibbstown, NJ, USA). Since all the fruit vinegar samples were colored, 2 mL of butanol (Sigma-Aldrich Ltd., Oakville, ON) was added to each sample to separate the pink chromogen from fruit pigments. After adding butanol, samples were centrifuged at 2000 g for 15 minutes. Florescence was measured in an aliquot of butanol fraction using the 96-well FLUOstar OPTIMA micro-plate reader at 535 nm and 590 nm excitation and emission wave lengths respectively. TBARS activity was expressed as the percent inhibition of LDL oxidation, compared to the positive control.
Angiotensin Converting Enzyme (ACE) Inhibition Assay
The ACE inhibitory activity of fruit vinegar beverages was performed based on the studies of Cinq-Mars and Li-Chan [15] and Santos et al. [16] and according to the method described by Balasuriya and Rupasinghe [17] with some modifications. Twenty one micro liters of sample were slowly mixed with 150 μL the substrate HHL (Sigma Aldrich Canada Ltd. Oakville, ON, Canada). Thirty micro liters of ACE (ACE extracted from rabbit lung, Sigma Aldrich Canada Ltd. Oakville, ON, Canada) was added to each tube, mixed and incubated at 37°C using a shaker oven (Model: HP 50, Apollo Instrumentation for Molecular Biology, CA, USA) for 1 hour. After 1 hour incubation period 0.35 M NaOH (Sigma Aldrich Canada Ltd. Oakville, ON, Canada) 150 μL was added to each tube to stop the enzymatic reaction. Then, 100 μL of O-phald ialdehyde (Sigma Aldrich Canada Ltd. Oakville, ON, Canada) was added and experimental units were kept at room temperature for 15 minutes to develop the fluorescent adduct. After 15 minutes, 3 M HCl (Sigma Aldrich Canada Ltd. Oakville, ON, Canada) 50 μL were added to terminate the reaction and the experimental units were loaded to 96-well microplates (COSTAR 3915, Fisher Scientific, Ottawa, ON, Canada). Fluorescent readings were taken at excitation and emission wavelengths of 360 nm and 500 nm respectively using the FLUOstar OPTIMA plate reader. Values were expressed as percent inhibition of enzyme with comparison to the positive control.
Statistical Analysis
The design for all experiments was a completely randomized design. Data was analyzed using the analysis of variance (ANOVA) using the general linear model (GLM) [18]. Assumptions of normality of error terms were tested using the Anderson-Darling test. Assumptions of constant variance were checked by plotting residuals versus fitted scatter diagram [18]. Differences among means were tested by the Tukey’s studentized range test at the level of p < 0.05 [19]. Each experiment was consisted of three replicates and each experiment was conducted independently three times. Results were expressed as means ± their standard deviations (n=9).
Results
Total Antioxidant Capacity
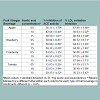
Four different fruit vinegar beverages were tested at three different levels of acidity for their total antioxidant capacities (Table 1) using FRAP and ORAC assays. When the acetic acid concentration increased from 5 to 15 mg acetic acid equivalents/g, apple and tomato vinegar beverages showed a significant difference (p < 0.05) in antioxidant capacity measured by FRAP assay. However, in the ORAC assay, all the other vinegar beverages except tomato showed a significant difference (p < 0.05) in antioxidant capacity when acetic acid levels increased. Furthermore, among four different vinegar beverages, tomato showed the lowest antioxidant capacity measured by both antioxidant assays and blueberry showed the highest.
Table 1: Total antioxidant capacity of different fruit vinegar beveragesp. Mean values ± standard deviation (n=9). qmg acetic acid equivalents/g. rlog transformation was done to obtain normality of data for ORAC assay. FRAP - Ferric Reducing Antioxidant Power. ORAC - Oxygen Radical Absorbance Capacity. TE - Trolox equivalents. a-cMeans followed by a different letter within each column for each type of fruit beverage is significantly different (p < 0.05).
Discussion
Fruits and vegetables are known to be a good source of antioxidants and biologically active polyphenol compounds. The complex mixture of phytochemicals present in fruits and vegetables provides protective health benefits, mainly through an additive and/or synergistic effect [2]. Furthermore, these polyphenol compounds have been found to exhibit strong antioxidant properties, both in vitro and in vivo [1]. Since fruits and vegetables are high in bioactive compounds, a diet containing fruits and vegetables could help prevent oxidative stress, prevent chronic disease and slow the aging process [20].In the current study, four different vinegar beverages: apple, cranberry, blueberry and tomato, were tested in vitro for their antioxidant and antihypertensive properties. Cranberry and blueberry vinegar beverages showed the highest antioxidant capacities measured by FRAP and ORAC assays. Blueberries have demonstrated relatively higher antioxidant capacities where 45 g of blueberry powder exhibited an antioxidant capacity of 16.0 mmol TE/L as measured by ORAC [21]. Cranberries have also reported higher antioxidant activity (0.177 mmol vitamin C equivalents/g of fresh weight) than apples (0.098 mmol vitamin C equivalents/g of fresh weight) [2]. Furthermore, Sablani et al. [22] demonstrated that total antioxidant activity of blueberries ranged between 9.1 and 16.9 mmol Trolox equivalents per kg fresh weight. Anthocyanins are the major bio-active group of compounds present in cranberries and blueberries and have been well documented for their antioxidant properties. Data of the current study was in agreement with the previous findings where, blueberry and cranberry vinegar beverages showed better antioxidant capacities in vitro compared to apple and tomato vinegar beverages. It was reported that blanching treatment prior to processing of blueberries aided retaining higher levels of total anthocyanins, phenolics and antioxidant activity [22]. The manufacturer (Van Dyke, Caledonia, Queens Co., NS, and Canada) where the blueberry juice was purchased practice blanching prior to processing the blueberry juice and therefore, can in turn enhance the antioxidant properties of the blueberry vinegar beverage.
Chronically elevated LDL levels and their oxidative modifications have been known to be leading causes of atherosclerotic plaque formation [23]. in vitro LDL oxidation has been commonly used by researchers as a model to understand the antioxidant ability of test products or compounds. In this context, fruits and fruit products which are rich sources of numerous bioactive compounds have been tested for their in vitro LDL oxidation inhibition ability [14,24]. Results of the currents study revealed that blueberry and cranberry vinegar beverages have shown greater in vitro LDL oxidation inhibition in comparison to other two vinegar beverages regardless the concentration of acetic acid in the beverages. On the other hand, LDL oxidation inhibition by apple and tomato vinegar beverages was dependant on the acetic acid concentration. As the results illustrate, higher the juice content, greater the oxidation inhibition. Although, tomato vinegar beverage showed less LDL oxidation inhibition in the in vitro system in the current study, tomato bio-active compounds, lycopene in particular have shown promising in vivo effects in atherosclerosis prevention [25]. As reviewed by Thilakarathna and Rupasinghe [14], lycopene can be effective in numerous ways other than inhibiting oxidation of LDL: reducing numerous inflammatory biomarkers, reducing serum total cholesterol levels, inhibiting the rate limiting enzyme HMG-CoA reductase.
To the best of our knowledge, reports on LDL oxidation inhibition by vinegar beverage are scarce. However, there are numerous findings on in vitro LDL oxidation inhibition by different fruit juices. A study revealed that commercial brands of fruit juices inhibited in vitro LDL oxidation at a range of 9 to 34%, whole apples and apple peels by 34% and flesh alone by 21% [26]. A study carried out to compare antioxidant potency of commonly consumed beverages reported that blueberry and cranberry juiced were better in terms of in vitro LDL oxidation inhibition as well as total polyphenol content [27]. These findings are in line with the findings of the current study. Vinegar beverages have previously shown to inhibit in vitro LDL oxidation where balsamic vinegar significantly prolonged the LDL oxidation lag time and reduced TBARS formation in HUVEC-mediated LDL oxidation [28]. The authors’ further investigations on human subjects confirmed that the inhibition of LDL oxidation and oxidized-LDLinduced foam cell formation was by decreasing the expression of scavenger receptors in macrophages.
ACE plays a major role in reducing the blood pressure by controlling the over activation of rennin angiotensin aldosterone system. Although there are numerous antihypertensive drugs available in the market today, natural ACE inhibitors continue to attract the attention. Literature has reported the ACE inhibition ability of plant extracts rich in flavonoids, especially berries and apples [29]. In the current study, cranberry and blueberry have shown better inhibition of ACE in vitro, in comparison to the other two vinegar beverages. Furthermore, it was found that anthocyanins and plant extracts rich in anthocyanins exhibited better ACE inhibition in both in vitro and in vivo model systems [30]. The cyanidin molecule was known to play a major role in ACE inhibition. Greater inhibition of ACE in vitro, by blueberry and cranberry vinegar beverages compared to the other two beverages confirms these previous findings.
The present study verified that functional fruit vinegar beverages had a hypotensive and hypolipidemic effects in vitro. Thus, above results indicate that the fruit vinegar beverages could be used as an alternative drug or as a functional beverage in controlling the blood pressure and serum cholesterol levels in the body. Further investigation on the antioxidant capacities and antihypertensive activities of berries, apples and tomatoes and their mechanisms of action are important related to the promotion of human health and disease prevention. However, to support the research finding of the in vitro results, further research involving experimental animal models and human clinical trial are warranted.
Acknowledgements
The authors acknowledge Dr. Li Juan Yu for her support and Atlantic Innovation Fund (AIF) program of the Atlantic Canada Opportunity Agency (ACOA), for providing the financial support for this research.References
- Hurst RD, Wells RW, Hurst SM (2010) Blueberry fruit polyphenolics suppress oxidative stress induced skeletal muscle cell damage in vitro. Mol Nutr Food Res 54: 353-363.
- Sun J, Chu YF, Wu X, Liu RH (2002) Antioxidant and anti-proliferative activities of common fruits. J Agric Food Chem 50: 7449-7454.
- Lohachoompol V, Srzednicki G, Craske J (2004) The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotechnol 5: 248-252.
- Wang L, Liu S, Manson JE, Gaziano JM, Buring JE, et al. (2006) The consumption of lycopene and tomato-based food products is not associated with the risk of type 2 diabetes in women. J Nutr 136: 620-625.
- Chang RC, Lee HC, Ou SM (2005) Investigation of the physicochemical properties of concentrated fruit vinegar. J Food Drug Anal 13: 348-356.
- Natera R, Castro R, Moreno MDVG, Hernandez MJ, Barroso CG (2003) Chemometric studies of vinegars from different raw materials and processes of production. J Agric Food Chem 51: 3345-3351.
- Bastante MJC, Guerrero E, Meji´as RC, Mari´n RN, Dodero MCR, et al. (2010) Study of the polyphenolic composition and antioxidant activity of new sherry vinegar-derived products by maceration with fruits. J Agric Food Chem 58: 11814-11820.
- Su MS, Chien PJ (2010) Aroma impact components of rabbiteye blueberry (Vaccinium ashei) vinegars. Food Chem 119: 923-928.
- Ahmet I, Spangler E, Hale BS (2009) Blueberry-enriched diet protects rat heart from ischemic damage. PLoS One 4: e5954.
- Lam CK, Zhang Z, Yu H, Tsang SY, Huang Y, et al. (2008) Apple polyphenols inhibit plasma CETP activity and reduce the ratio of non-HDL to HDL cholesterol. Mol Nutr Food Res 52: 950-958.
- Engelhard YN, Gazer B, Paran E, Sheva B (2006) Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am Heart J 151: 100.
- Nakamura K, Ogasawara Y, Endou K, Fujimori S, Koyama M, et al. (2010) Phenolic compounds responsible for the superoxide dismutase-like activity in high-brix apple vinegar. J Agric Food Chem 58: 10124-10132.
- Rupasinghe HPV, Wang L, Huber GM, Pitts NL (2008) Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem 107: 1217-1224.
- Thilakarathna SH, Rupasinghe HPV, Needs PW (2013) Apple peel bioactive rich extracts effectively inhibit in vitro human LDL cholesterol oxidation. Food Chem 138: 463-470.
- Cinq-Mars CD, Li-Chan EC (2007) Optimizing angiotensin 1-converting enzyme inhibitory activity of pacific hake (Merluccius productus) fillet hydrolysate using response surface methodology and ultrafiltration. J Agric Food Chem 55: 9380-9388.
- Santos RA, Krieger EM, Greene LJ (1985) An improved fluorometric assay of rat serum and plasma converting enzyme. Hypertension 7: 244-252.
- Balasuriya NBW, Rupasinghe HPV (2012) Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem 135: 2320-2325.
- Montgomery DC (2005) Design and Analysis of Experiments. (6th edition), John Wiley and Sons, Hoboken, New Jersey.
- SAS Institute Inc (2008) SAS User’s Guide: Statistics Version 9.2, SAS Institute Inc, Cary, NC, USA.
- Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutrition Journal 3: 1475-1490.
- Stull AJ, Cash KC, Johnson WD (2010) Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 140: 1764-1768.
- Sablani SS, Andrews PK, Davies NM (2010) Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. J Sci Food Agric 90: 769-778.
- Barter P (2005) The inflammation: lipoprotein cycle. Atheroscler Suppl 6: 15-20.
- Chu YF, Liu RH (2005) Cranberries inhibit LDL oxidation and induce LDL receptor expression in hepatocytes. Life Sci 77: 1892-1901.
- Thilakarathna SH, Rupasinghe HPV (2012) Anti-atherosclerotic effects of fruit bioactive compounds: A review of current scientific evidence. Can J Plant Sci 92: 407-419.
- Pearson D, Tan C, German B, Davis P, Gershwin M (1999) Apple juice inhibits low density lipoprotein oxidation. Life Sci 64: 1919--1920.
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, et al. (2008) Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem 56: 1415-1422.
- Iizuka M, Tani M, Kishimoto Y, Saita E, Toyozaki M, et al. (2010) Inhibitory effects of Balsamic vinegar on LDL oxidation and lipid accumulation on THP-1 macrophages. J Nutr Sci Vitaminol 56: 421-427.
- Balasuriya NBW, Rupasinghe HPV (2011) Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Functional Foods in Health and Diseases 5: 172-188.
- Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, et al. Inhibition of angiotensin converting enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol 127: 7-10.