Journal of Emergency Medicine & Critical Care
Download PDF
Research Article
Incidence of Pulmonary Embolism in Patients with Positive D-Dimers and Chest X-ray Evidence of Pneumonia: A Retrospective Study
Matthew Hysell* and Blake Collier
- Michigan State University, Lakeland Healthcare, Department of Emergency Medicine, St. Joseph, Michigan, USA
*Address for Correspondence: Matthew Hysell, Michigan State University, Lakeland Healthcare, Department of Emergency Medicine, St. Joseph, Michigan, USA, E-mail: matthysell@comcast.net
Citation: Hysell M, Collier B. Incidence of Pulmonary Embolism in Patients with Positive D-Dimers and Chest X-ray Evidence of Pneumonia: A Retrospective Study. J Emerg Med Critical Care 2018;4(1): 3.
Copyright © 2018 Hysell M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Emergency Medicine & Critical Care | ISSN: 2469-4045 | Volume: 4, Issue: 1
Submission: 08 June, 2018 | Accepted: 10 July, 2018 | Published: 19 July, 2018
Abstract
Introduction: Evaluation of chest pain in the Emergency Department is common. Significant resources are expended looking for dangerous etiologies. The D-dimer is frequently utilized but can be positive in a variety of pathologic and non-pathologic states, including pneumonia. We anticipated that patients who had pneumonia on chest x-ray and also a positive D-dimer would have a low likelihood of also having pulmonary embolism. We hoped to define this patient population as low risk of having PE in the setting of pneumonia with the purpose of limiting unnecessary CT angiographies.
Methods: We performed a retrospective analysis to identify patients who had an elevated D-dimer, evidence of pneumonia by chest x-ray and who underwent subsequent CT angiography [CTA] or Ventilation/perfusion [V/Q] scanning. We correlated the results of the CTA or V/Q with patient demographics, vital signs, and laboratory values to evaluate our patient population.
Results: We identified 151 patients who had an infiltrate on the chest x-ray and elevated d-dimer that subsequently went on to have CTA or V/Q to rule out pulmonary embolism. Of this group of patients 7/151 [4.6%] had a PE. We then performed statistical analysis using the vital signs, lab values, and patient demographics to look for differences between patients with pulmonary embolism and without. However, no statistically significant conclusions could be made.
Conclusions: In patients with elevated D-dimer and pneumonia our series demonstrated a small but not uncommon rate of concurrent PE. A larger study group would be required to determine risk stratification of this group.
Introduction
Evaluation of chest pain and difficulty breathing in the Emergency Department [ED] is commonly faced by Emergency Providers [EP’s]. Accounting for about 5% of all ED visits, significant resources are expended looking for dangerous causes of chest pain [1]. Although classically the clinical descriptions of pneumonia and pulmonary embolism are quite different, clinically these two important diagnoses have a considerable amount of overlap. During the initial ED evaluation of acutely-ill patients with chest pain or difficulty breathing vital signs, basic laboratory work up, and simple imaging with chest x-rays often guide further workup and management. The D-dimer is a common (and often controversial) lab test utilized for screening for pulmonary embolism when the initial diagnosis is not easily apparent [2]. Because the D-dimer is very non-specific, it can be positive at various levels in a variety of pathologic and non-pathologic states. As a result of considering a broad differential diagnosis during the initial work up it is not uncommon for a patient to have a chest x-ray that is concerning for a pneumonia and an elevated D-dimer. Although recent studies have shown that pneumonia is a common cause for elevated D-dimer and that D-dimer has prognostic value in some but not all series of patients with pneumonia, in clinical practice these patients often undergo further pulmonary CT angiography to evaluate for PE [3-6]. We hypothesized that only a small subset of the population would have both pneumonia on chest x-ray and pulmonary embolism on CTA and could perhaps be spared further work-up and reducing iatrogenic events and healthcare costs for the patient.
Methods
Study design
This study used a retrospective analysis using EPIC Electronic Health Records to identify adults presenting to the emergency department who had an elevated D-dimer, evidence of pneumonia by chest x-ray, and who underwent subsequent CTA or V/Q. The study was reviewed by our institutional IRB and deemed exempt from formal consent due to its retrospective and non-interventional nature.
Study setting and population
The patient encounters were extracted from March 2012 to June 2015 presenting at two community hospitals in southwest Michigan. Both community hospitals are part of a single hospital system.
Study protocol
Using Microsoft SQL Server, patients with an elevated D-dimer >230 ng/mL, and who had undergone both initial CXR as well as PE protocol CTA or V/Q were identified and radiology results extracted. We reviewed these results and identified patients with an “infiltrate” or having frank diagnosis of “pneumonia” on formal radiology interpretation. Due to the retrospective nature of the study we did not attempt to determine providers’ working diagnosis prior to CTA or V/Q with regards to differential for an infiltrate. Chest x-rays that were equivocal, i.e. “infiltrate vs. atelectasis” were not included in our study. During the time period data was abstracted, a positive D-dimer was considered >230 ng/mL. From this initial group of patients, we then identified those who had explicit diagnosis of PE on subsequent CTA or V/Q scan. We also included patients who had equivocal CTA or V/Q results for PE, but whose providers treated clinically for PE. CTA results were considered equivocal when there were mentions of “filling defects” without explicitly endorsing the diagnosis for PE, “probable embolus”, or “suggestive of embolism”.
Data analysis
For all patients who met the inclusion requirements age, sex, vital signs at presentation to the ED (including heart rate, blood pressure, and temperature), white blood cell count (WBC), troponin, and D-dimer level were extracted. Chi-squared analysis and t-test were then calculated to determine what, if any, clinically significant conclusions could be made.
Results
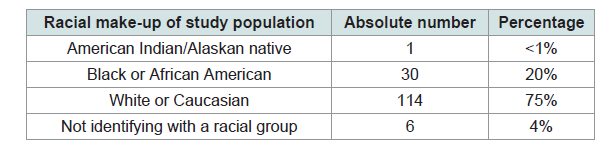
Our retrospective chart review identified 2287 patients with an elevated D-dimer, an initial chest x-ray as well as a follow-up confirmatory study for PE. We identified a total of 151 patients who had an initial chest x-ray with evidence of pneumonia. Racial make-up of the study population is Table 1.
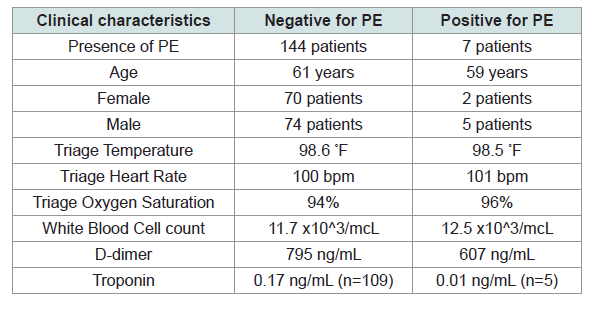
Of these 151 patients, 7 patients were later found to have a pulmonary embolism while 144 had negative work ups for pulmonary embolism. Results of the clinical variables extracted are in Table 2.
Discussion
Elevated D-dimer testing is commonly encountered in the work-up of ED patients with chest pain and/or difficulty breathing. We found that nearly 5% of patients who could easily have been diagnosed with pneumonia actually had a PE in the presence of an elevated D-dimer. Castro DJ et al. found that both pneumonia and pulmonary embolism (PE) produced an elevated D-dimer (though PE averaged higher, and significantly so in high-probability PE cases). Because of overlap, however, they conclude that D-dimer is not a helpful test to screen for PE in the setting of pneumonia.
More recent studies focusing on D-dimer in pneumonia have attempted to use it as a risk-stratification tool, with some success [7-9]. However, these studies investigated patients already carrying the diagnosis of community-acquired pneumonia. Querol-Ribelles JM et al. measured D-dimer on 302 patients over age 16 with opacities on chest x-ray [CXR] with an infectious syndrome, excluding those with a high wells probability of PE [2,7]. They found that 85% of patients with PNA had a positive D-dimer and that non-survivors had, on average, a D-dimer double that of survivors (also statistically significant). Chalmers JD et al. measured D-dimer on 314 adults with a new infiltrate on CXR in patients with 3 of cough, sputum, breathlessness, pleurisy, hemoptysis, fever, headache, or exam findings consistent with pneumonia, excluding any patients with known PE [8]. They compared negative D-dimer to CURB-65 and Pneumonia Severity Index, finding that it was comparable and may identify some patients as low risk that the other models would identify as higher risk (these patients did well) [10,11]. Arslan S et al. evaluated 60 patients diagnosed with lobar or multi-lobar pneumonia, also finding that D-dimer increased with severity of illness, as well as in the presence of co-morbid conditions [9].
What all of the above studies have in common, however, is that they are evaluating D-dimer’s ability to predict pneumonia severity in patients already carrying the diagnosis. Zhang Z et al. looked at 139 patients carrying the diagnosis of community-acquired pneumonia who had elevated D-dimers and who underwent CT angiography [12]. Fully 66% were diagnosed with PE in their series. They found troponin-I to be elevated significantly more frequently in those with PE. Our study found (non-significantly) the opposite. In clinical practice, however, D-dimer is not used widely for pneumonia scoring, and has not yet been validated for use in this fashion. Another reason it has not gained acceptance is likely clinicians’ fear of introducing diagnostic uncertainty given D-dimer’s more widespread use in screening for PE. While pneumonia should be recognized as a common cause of elevated D-dimer, in clinical practice patients with both positive CXR’s and positive D-dimers frequently undergo pulmonary CT angiography to look for PE. In fact, our study demonstrated a small (4.6%) but not uncommon rate of pulmonary embolism. This is a higher rate than we had hoped; the decision to forego further work-up for PE would require shared decision-making with the patient.
We reviewed a number of clinical variables in terms of both lab values and vital signs to look for trends suggesting which patients might have underlying PE or confirm pneumonia. Overall in our patient population the patients with pneumonia averaged very slightly (and non-significantly) worse lab results and vital signs for the most part. Interestingly the PE patients averaged higher WBC count and lower D-dimer than those who did not have PE. Our sample size was too small to make any statistically significant predictors of which patients are at high risk and need a CTA to further rule out pulmonary embolism in the setting of an elevated D-dimer and pneumonia based on CXR. Paparoupa M et al. performed a case control study on 100 patients, approximately half with PE in addition to pneumonia and the other half without PE and were also unable to determine a prediction rule [13].
Limitations
This study is subject to several limitations. As a retrospective study our analysis was limited due to the variables that could be identified using the electronic health record. As a retrospective study, we were reliant on radiology reads by the radiologist on-duty at the time. The radiology reads, including chest x-rays, were often ambiguous and only patients with a definitive diagnosis of pneumonia were included in the study. The study was also performed at a single hospital system from two different community hospitals and study population was dominated by 2 racial groups. Our study was too small to assess for differences in the clinical variables we reviewed. In addition, there were numerous CTA results that were equivocal for PE. We only included patients in our study that were treated as having a PE and had a discharge diagnosis of PE. The differences in provider care, whether aggressive or conservative in nature, may have lead to an unrecognized bias in our study as this does not account for whether there was a high or low likelihood of PE.
Conclusion
Nearly 5% of our patients with an infiltrate consistent with pneumonia and a positive D-dimer did in fact have a PE, likely too high of a rate to forego definitive testing for PE. While we did not demonstrate any statistically significant difference between those who had pulmonary embolism and those who were diagnosed with pneumonia we present these data as they may be useful for further meta-analysis.
References
- Green GB, PM Hill (1996) Approach to chest pain and possible myocardial ischemia. In: Cline DM, Ma JO, Tintinalli JE, et al. (Eds) Emergency medicine - a comprehensive study guide (4thedn), Companion Handbook. McGraw-Hill Publications, New York, USA, pp. 341.
- Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, et al. (1998) Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 129: 997-1005.
- Castro DJ, Pérez-Rodríguez E, Montaner L, Flores J, Nuevo GD (2001) Diagnostic value of D dimer in pulmonary embolism and pneumonia. Respiration 68: 371-375.
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699-709.
- Zhang XL, Wang Z, Lv SH, Jing HJ, Kang JY, et al. (2016) C reactive protein, calcitonin and D-dimer in patietns of community acquired pneumonia. J Hainan Med Univ 22: 27-29.
- Duarte J, Castro AT, Silva R, Correia L, Simao A, et al. (2015) Prognostic value of plasma D-dimer level in adults with community -acquired pneumonia-a prospective study. European Respiratory J 46: 2578.
- Querol-Ribelles JM, Tenias JM, Grau E, Querol-Borras JM, Climent JL, et al. (2004) Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest 126: 1087-1092.
- Chalmers JD, Singanayagam A, Scally C, Hill AT (2009) Admission D-dimer can identify low-risk patients with community-acquired pneumonia. Ann Emerg Med 53: 633-638.
- Arslan S, Ugurlu S, Bulut G, Akkurt I (2010) The association between Plasma D-dimer levels and community-acquired pneumonia. Clinics (Sao Paulo) 65: 593-597.
- Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, et al. (2003) Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58: 377-382.
- Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, et al. (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336: 243-250.
- Zhang Z, Zhou Q, Zou Y, Song X, Xie S, et al. (2015) Risk factors for pulmonary embolism in patients preliminarily diagnosed with community-acquired pneumonia: a prospective cohort study. J Thromb Thrombolysis 41: 619-627.
- Paparoupa M, Spineli L, Framke T, Ho H, Schuppert F, et al. (2016) Pulmonary embolism in pneumonia: Still a diagnostic challenge? Results of a case-control study in 100 patients. Dis Markers 2016: 8682506.