Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Comparison of the Efficacy of Topical Human Breast Milk versus Hydrocortisone 1% Lotion in the Clinical Improvement of Atopic Eczema in Infants: A Non-inferiority Trial
Erica T. Rogando-Tan1*, Elizabeth Amelia V. Tianco2, Daisy King-Ismael2 and Deana Dabay-Tan3
- Department of Dermatology, Jose R. Reyes Memorial Medical Center, Manila, Philippines
- Consultant Dermatologist, Jose R. Reyes Memorial Medical Center, Manila, Philippines
- Consultant Dermatologist, Novaliches General Hospital, Quezon City, Philippines
*Address for Correspondence: Erica T. Rogando-Tan, Department of Dermatology, Jose R. Reyes Memorial Medical Center, Manila, Philippines, E-mail: ericarogando@yahoo.com
Citation: Tan ET, Tianco EA, Ismael DK, Tan DD. Comparison of the Efficacy of Topical Human Breast Milk versus Hydrocortisone 1% Lotion in the Clinical Improvement of Atopic Eczema in Infants: A Non-inferiority Trial. J Clin Investigat Dermatol. 2018;6(1): 5.
Copyright © 2018 Tan ET, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical & Investigative Dermatology | ISSN: 2373-1044 | Volume: 6, Issue: 1
Submission: 24 January, 2018 | Accepted: 22 February, 2018 | Published: 28 February, 2018
Introduction
Atopic Dermatitis (AD) is an inflammatory skin disease associated with pruritus and inflammation due to interactions between genetic susceptibility genes, resulting in a defective skin barrier and heightened immunologic responses to environmental allergens and microbial antigens. Approximately 15-20% of infants are affected by AD in developed countries and about 50% of them have atopic eczema in the first years of their lives [1]. Highly prevalent in early childhood, AD is exemplified by its chronic recurrence of erythema, excoriations, erosions and pruritus which makes it a global disease burden affecting most aspects of a person’s daily life [2].
A stepwise approach in the treatment of AD in children is recommended by the National Institute for Health and Clinical Excellence (NICE). This includes education, use of emollients, avoidance of triggering factors, topical corticosteroids, topical calcineurin inhibitors and systemic immunosuppressive therapy. Topical glucocorticoids still remain as the cornerstone of treatment for the inflammatory eczematous skin lesions in AD [3]. Certain topical corticosteroids such as hydrocortisone and mometasone have been approved for use in infants [4]. However, its excessive and long-term use may have adverse effects including cutaneous irritation, burning, itching, steroid atrophy, steroid acne and striae distensae [5]. Moreover because many caregivers have steroid phobia which in turn affects adherence to the medications, alternative treatment for infantile AD is of interest because of the chronic recurrent nature and area of predilection, affecting sensitive areas such as the face. For infantile rash there is a higher demand for alternative treatment options that are natural and non-steroidal with better side-effect profile. Human Breast Milk (HBM) has been analyzed for its various medicinal properties. HBM’s specialized immune components have exhibited antimicrobial properties that are responsible for HBM’s notable efficacy in decreasing antimicrobial colonization in vitro [6-8]. In developing countries it has also been proven to be effective in the treatment of conjunctivitis cause by Chlamydia [9]. Mohammadzadeh et al. have shown that it is comparable to lanolin in improving nipple eczema in breastfeeding women [10].
In current practice, public health midwives and lactation consultants have been recommending the topical use of HBM as treatment for infantile rash. This is supported by an increasing number of published researches validating the anti-inflammatory property of HBM in infantile skin conditions such as diaper dermatitis and atopic eczema [11-15].
Increasing evidence from these published researches also support the anti-inflammatory property of HBM. A study done my Farahani et al. comparing the effect of human milk and topical hydrocortisone 1% on diaper dermatitis, showed that treatment with HBM was as effective as hydrocortisone 1% ointment in reducing severity scores in seven days [13]. This was attributed to milk components that directly exert an anti-inflammatory effect or indirectly create unfavorable environment conditions for bacterial growth by modifying the commensal flora the pH or bacterial substrates [13]. Lactation consultants have been advocates in promoting topical HBM application on sore nipples and infantile rash [13]. Mohammadzadeh et al. has shown that it is comparable to lanolin in improving sore nipples in breastfeeding women [10]. It was postulated that human milk being a source of two classes of major growth factors, transforming growth factors alpha (TGF-α) and beta (TGF-β) and the Insulin-like Growth Factors (IGF) may have roles in wound healing. TGF-α and TGF-β are involved in normal cell activities such as embryonic development, cell proliferation and tissue repair.
A randomized controlled trial was done by Kasrae et al. comparing expressed human milk versus hydrocortisone in infants with mild to moderate AD using the objective SCORAD severity index and the patient-oriented scoring atopic dermatitis index. The study showed that the degree of improvement in the infants skin condition was similar between the two groups at day 7, 14 and 21 [16]. A clinical appraisal of this article by Ng et al. was published in the Journal of the Philippine Dermatological Society concluded that there is sufficient evidence based on the article to say that HBM is comparable to hydrocortisone 1% ointment in terms of efficacy, in healing lesions of AD in infants [17].
The significance of this study rests on the search for alternative treatment options that are readily available, safe, and cost-effective with no long-term side effects for the clinical improvement of infantile AD. To the best of our knowledge this study is the first to
compare hydrocortisone and HBM using an objective parameter of AD severity by measuring transepidermal water loss, instead of only using subjective parameters seen in other AD severity scoring indices.
Objectives of the study
The general objective of the study was to determine the efficacy of topical HBM compared to topical hydrocortisone 1% in the clinical improvement of infantile AD.
The specific objectives were to determine if outcomes in patients treated with HBM differed significantly from patients treated with hydrocortisone 1% in terms of
- The proportion of patients with moderate or excellent improvement based on EASI scores.
- Post-treatment change from baseline in mean total Eczema Area and Severity Index (EASI) scores; mean Total Transepidermal Water Loss (TEWL) levels.
- Mean Infants Dermatitis Quality of Life Index (IDQLI) scores.
Methodology
Participants and study design
A randomized controlled trial was used to determine whether HBM is comparable to hydrocortisone 1% lotion in the clinical improvement of AD in patients with aged 2-24 months old who were diagnosed at the Out-Patient Dermatology Department of Jose R. Reyes Memorial Medical Center Department from March to August 2017.
A certificate of approval from the Institutional Review Board of the hospital was obtained before conducting the study. Assent form in the vernacular was also secured from the patient’s guardian.
This non-inferiority randomized trial aimed to offer data and evidence to support the use of topical HBM as a comparable alternative to hydrocortisone in treatment of inflammatory lesions of infantile atopic dermatitis [18-21].
Materials
For the treatment arm, mothers were taught to hand express milk to be applied onto lesions covering the entire area affected. In the control arm, hydrocortisone 1 % lotion 100 ml (Formulated by Dermskin, Manila) was provided for application onto lesions. Both treatment and control groups were provided with mild soap (DoveTM; Unilever PLC, London, UK) and commercially available emollient HyalureTM hypoallergenic lotion; Manila, PH).
Study subjects
The subjects included in the study were patients 2-24 months of age who were newly or previously diagnosed as mild to moderate AD based on an EASI score of less than or equal to 50. These patients were selected based on criteria of being breastfed (Exclusively or mixed with formula feeding) and able to follow up at the outpatientdepartment.
Excluded from the study were patients who had topical or oral antibiotic or steroid treatment in the two weeks prior to enrollment, patients who required systemic therapies for flares and/or maintenance, patients with grossly infected lesions that required oral or intravenous antibiotics and ancillary therapy, patients with dermatologic diagnoses other than AD, patients with congenital anomalies and those with oral or genital thrush. In addition study participants should not have an allergy to hydrocortisone and should not use any other medications that might affect the results of the study.
Study intervention
Infants who met the entry criteria were randomly assigned to either the topical application of HBM or hydrocortisone 1% lotion. The co-investigators, study coordinator and statistician were blinded from the study. The study statistician generated a list of random numbers using the random number generator from MegaStat (version 10.4) software. An assigned resident who was blinded to the codes, will allocated the treatments.
In the topical HBM group the subjects mothers were instructed to hand express their milk twice a day and apply it on to the affected area making sure to cover the entire lesions defined the investigator at the baseline. In the hydrocortisone 1% group a thin layer of the lotion was applied twice a day to all areas of the actively diseased skin as defined by the investigator at the baseline. All parents also received general instructions about the infants AD care, prevention of trigger factors such as extreme temperature environments and keeping nails short to minimize skin scratching. This intervention was patterned on the study done by Kasrae et al. comparing topical HBM and hydrocortisone on AD among infants [16].
Clinical assessment
The infants were evaluated on the first day (baseline) and weeks 1, 2 and 3 of the treatment. Severity of AD was measured using EASI, objective Transepidermal Water Loss (TEWL) levels and patient-oriented Infant-Dermatology Quality of Life Index (IDLQI) questionnaire.
During the first visit with the parents the parents of the patients were interviewed on their demographic data, patient’s history of atopy, breastfeeding status and infant and maternal health. The research assistant who assessed the AD result based on EASI was blind to the interventions used on the affected areas. Another research assistant who measured the TEWL in the selected areas affected used the TewameterTM300 (Multiprobe Adapter MPA5) was also blinded from the intervention used. Each visit the parents answered an IDQLI questionnaire to serve as a patient-oriented subjective parameter tomeasure AD improvement.
Improvement based EASI and IDLQI scores were defined according to the following no improvement was an increase or <30% decrease from baseline EASI/DLQI, moderate improvement was a decrease of ≥30% but <70% EASI/DLQI and excellent improvement was a decrease of ≥70% EASI/DLQI. TEWL values were interpreted according to the instruction manual as of 0-10 (Very healthy condition), 10-15 (Healthy condition), 15-25 (Normal condition), 25-30 (Strained skin) and >30 (Critical condition).
Stopping guidelines
The trial was stopped if the patient was not be able to tolerate any side effects or has not shown any improvement after 14 days of treatment. Patients who failed to comply with the treatment assigned to the man who used topical medications other than the ones provided, were withdrawn from the study. If there were patients under the topical HBM group who did not improve after two weeks of treatment they were given hydrocortisone 1% lotion.
Sample size determination
The target sample size was based on a previous study done by Kasrae et al. showing 65.5% success rate in the hydrocortisone group and 75.9% success in the HBM group [16]. The maximum permissible error was set at 5 % with confidence level at 80 % [18]. The noninferiority margin (δ) was set at 20 %. Sample size was computed using the Power calculator for continuous outcome non-inferiority trial. [Online] Sealed Envelope Ltd 2012. Available from: https://www.sealedenvelope.com/power/continuous-noninferior/[Accessed Mar 2017]. The study aimed to recruit 28 patients in each study arm.
Data processing and analysis
For the descriptive analysis, Students t-test was used for continuous variables and Pearson’s chi-squared test was used for categorical data. Fisher’s exact test was used to determine if there was significant difference in the overall success rate between the two groups. ANOVA of repeated measures and post-hoc comparison were done to determine if there were significant differences between baseline and follow-up scores of EASI, IDQLI and TEWL levels of both groups at each time point. 95 % confidence interval was obtained for per protocol and intention-to-treat analysis to determine noninferioritywhere delta was set at 20.
Statistical analyses were performed using MegaStat (version 10.4) software. Test results that produced P-values of <0.05 were regarded as statistically significant.
Results
Study population
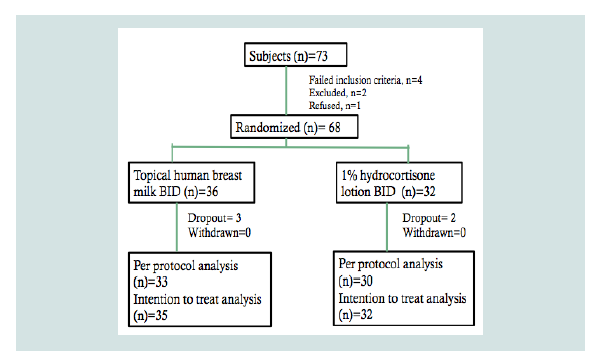
Of the 73 patients screened to participate from March to August 2017, four did not meet the inclusion criteria, two were excluded because their EASI scores were ≥50 and one refused to join the study because of inability to follow-up. Hence, 68 patients were included in the analyses. The patients were randomized to either the HBM arm or the topical 1% hydrocortisone arm (Figure 1). Under the topical HBM arm, a total of three dropouts were noted; one was lost to follow up and two were excluded because of parents’ failure to follow the instructions for applying assigned intervention. Under the hydrocortisone 1% arm, two were also lost to follow-up.
Figure 1: Flowchart of subjects with atopic dermatitis randomized to eithertopical HBM group or hydrocortisone 1% group.
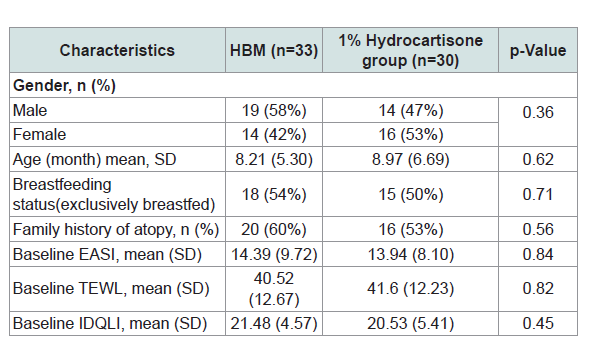
Table 1 shows the demographic and baseline characteristics ofthe subjects. Using chi-squared test for categorical data and t-test for difference in means the results showed that there were no significant differences among treatment groups for gender, age, breast feeding status, family history of atopy, EASI, TEWL and IDQLI scores.
Table 1: Demographics and baseline characteristics of the study participants and comparisons between topical application of HBM and hydrocortisone 1% treatment groups.
Clinical effects
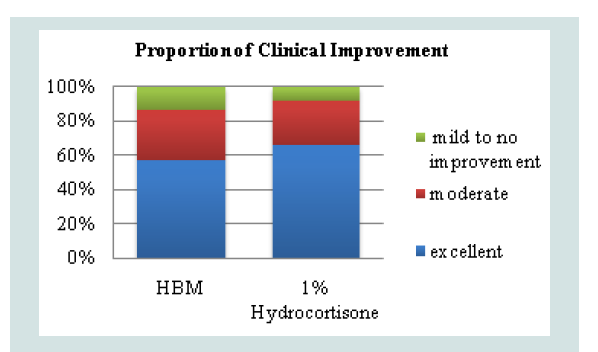
The overall success rate was derived from the proportion of patients who demonstrated clinical improvement, defined as moderate or excellent (Decrease in EASI score of ≥30%). A decrease of less than 30% or an increase in EASI score was considered as treatment failure. Fisher’s exact test was used to determine if there is a significant difference between the two groups based on treatment success. In the topical HBM intervention there were 19/33 (57%) who developed excellent improvement and 10/33 (30%) who developed moderate improvement. These were considered as treatment success. 4/33 (13%) were considered as treatment failures. Observations under hydrocortisone 1% intervention showed 20/30 (67%) to have excellent improvement, 8/30 (26%) moderate improvement and 2/30 (7%) treatment failure (Figure 2).
Figure 2: Proportion of patients who demonstrated clinical improvementbased on post-treatment EASI scores (p=0.4614).
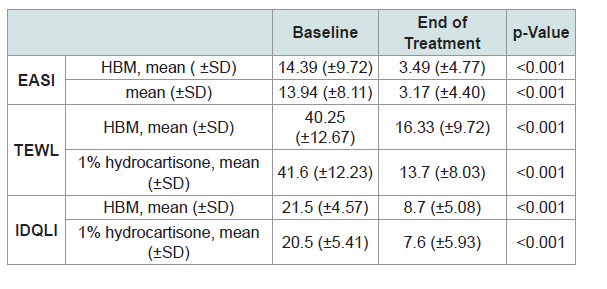
Comparison of mean EASI, TEWL and IDQLI scores revealed that baseline and post-treatment scores in the subjective, objective and patient-oriented scoring indices were significantly different, indicating efficacy of both interventions (p <0.001) (Table 2).
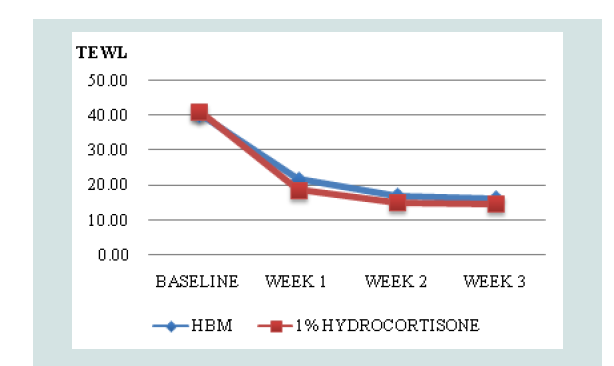
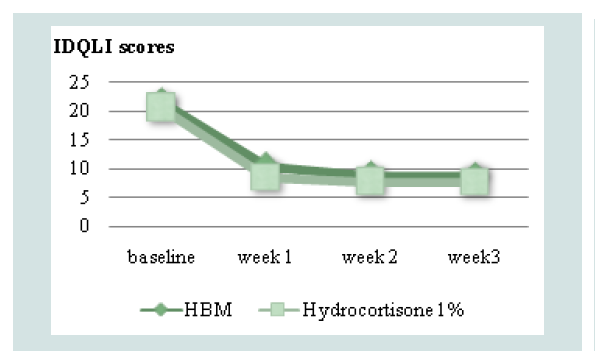
Comparison of mean TEWL and IDQLI scores at every point in time for the two groups, was done using ANOVA with post hoc comparison to further validate efficacy. In the HBM intervention there was a significant difference in the TEWL scores at baseline and all follow-ups (p<0.001) and at week 1 and 3 of follow up (p=0.0467). In the hydrocortisone 1% group, there was statistically significant difference in the TEWL scores at baseline and all follow-ups (p<0.001), at weeks 1 and 3 of follow up (p <0.001) and weeks 1 and 2 (p=0.0109). For the IDQLI scores, both groups showed statistically significant differences only comparing baseline to all follow-ups (p<0.001). However there were no significant differences seen between follow-ups (p>0.05) (Figure 3-5).
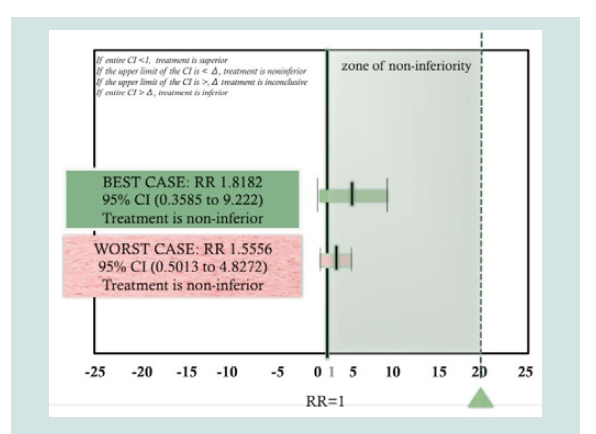
Based on per protocol analysis (Best-case scenario) the Relative Risk (RR) was computed to be 1.8182 with 95 % CI (0.3585 to 9.222). This excludes the non-inferiority threshold previously set at δ=20. Hence, treatment shows non-inferiority based on a per protocol approach. Using an intention-to-treat analysis, the computed RR computed was RR 1.5556 95% CI (0.5013 to 4.8272), this again excludes the non-inferiority margin hence treatment has still demonstrated non-inferiority using the intention-to-treat approach. Therefore, both per protocol and intention-to-treat analysis has shown non-inferiority of the topical HBM group compared to the hydrocortisone 1% group.
Discussion
This study showed that topical application of HBM is noninferior to hydrocortisone 1 % in the treatment of infants with mild to moderate AD. The clinical improvement based on subjective (EASI), objective (TEWL) and patient-oriented (IDQLI) scoreswere similar between the two groups. This validates the subjective findings by Kasrae et al. (From which this study was patterned to) that topical HBM was similar to hydrocortisone 1% in improving severity of AD using objective SCORAD severity index (OSSI) and patient-oriented SCORAD (PO-SCORAD) index [18]. This may be attributed to immunologic and anti-infective agents in HBM that act as natural antimicrobials and protective factors that provide specific and nonspecific passive immunity. A clinical appraisal of this article was published in the Journal of the Philippine Dermatological Society where in the authors concluded that there is sufficient evidence based on the article, to say that human breast milk is comparable to hydrocortisone 1% in terms of efficacy in healing lesions of AD in infants [19].
To further decrease bias of subjective findings in severity scoring, TEWL was measured to provide evidence of clinical improvement in this study. To our best knowledge, this is the first study to compare efficacy of HBM and hydrocortisone using TEWL. TEWL loss appears to be sensitive to disease expression throughout the lesional severity range of AD. The skin barrier which is damaged in patients with AD, results to disease amplification through increased per cutaneous absorption of antigens and irritants and subsequently a release in proinflammatory cytokines. TEWL serves as a direct measure and objective parameter of the permeability barrier of the skin which correlates to the healing and clinical improvement of the skin barrier [22]. TEWL was validated to be a sensitive indicator of fluctuations in AD disease activity compared to other validated and sensitive measures of AD severity such as SCORAD values, according to Chamlin et al. [23,24].
Conclusion
Topical HBM is non-inferior to hydrocortisone 1% lotion in the clinical improvement of mild to moderate atopic eczema in infants. Topical HBM twice a day has been shown to significantly decrease post-treatment severity scores (EASI, TEWL and IDQLI). It provides an alternative to topical corticosteroids in the treatment of infantile AD. A limitation of this study is the small sample size and that it is conducted only at a single-site which diminishes its external validity and generalizability. It is recommended that a larger scale study be done to improve the power of the study.
References
- Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 70: 338-351.
- Kliegman RM, Behrman ER, Jenson HB (2007) Nelson Textbook of Padiatrics, (18thedn). Philadelphia: Saunders, Elsevier.
- Lee BW, Detzel PR (2015) Treatment of childhood atopic dermatitis and economic burden of illness in Asia Pacific countries. Ann Nutr Metab 66 Suppl 1: 18-24.
- Zhao CY, Tran AQ, Lazo-Dizon JP, Kim J, Daniel BS, et al. (2015) A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol 173: 488-497.
- Ozon A, Cetinkaya S, Alikasifoglu A, Gonc EN, Sen Y, et al. (2007) Inappropriate use of potent topical glucocorticoids in infants. J Pediatr Endocrinol Metab 20: 219-225.
- Vural G, Kisa S (2006) Umbilical cord care: A pilot study comparing topical human milk, povidone-iodine, and dry care. J Obstet Gynecol Neonatal Nurs 35: 123-128.
- Ahmadpour-Kacho M, Zahedpasha Y, Hajian K, Javadi G, Talebian H (2006) The effect of topical application of human milk, ethyl alcohol 96%, and silver sulfadiazine on umbilical cord separation time in newborn infants. Arch Iran Med 9: 33-38.
- Amiri-Farahani L, Mohammadzadeh A, Tafazzoli M, Esmaeli H, Ghazvini K (2008) Effect of topical application of breast milk and dry cord care on bacterial colonization and umbilical cord separation time in neonates. J Chin Clin Med 3: 327-332.
- Chaumeil C, Liotet S, Kogbe O (1994) Treatment of severe eye dryness and problematic eye lesions with enriched bovine colostrum lactoserum. Adv Exp Med Biol 350: 595-599.
- Mohammadzadeh A, Farhat A, Esmaeily H (2005) The effect of breast milk and lanolin on sore nipples. Saudi Med J 26: 1231-1234.
- Tait P (2000) Nipple pain in breastfeeding women: causes, treatment, and prevention strategies. J Midwifery Womens Health 45: 212-215.
- Pugh LC, Buchko BL, Bishop BA, Cochran JF, Smith LR, et al. (1996) A comparison of topical agents to relieve nipple pain and enhance breastfeeding. Birth 23: 88-93.
- Farahani LA, Ghobadzadeh M, Yousefi P (2013) Comparison of the effect of human milk and topical hydrocortisone 1% on diaper dermatitis. Pediatr Dermatol 30: 725-729.
- Berents TL, Rønnevig J, Søyland E, Gaustad P, Nylander G, et al. (2015) Topical treatment with fresh human milk versus emollient on atopic eczema spots in young children: A small, randomized, split body, controlled, blinded pilot study. BMC Dermatol 15: 01-07.
- James WD, Berger TG, Elston DM (2016) Andrews’ Diseases of the Skin: Clinical Dermatology, (12thedn) Philadelphia, PA: Elsevier, pp. 965.
- Kasrae H, Amiri Farahani L, Yousefi P (2015) Efficacy of topical application of human breast milk on atopic eczema healing among infants: A randomized clinical trial. Int J Dermatol 54: 966-971.
- Ng JN, Bunagan MJ (2016) A critical appraisal of an article on therapy: Efficacy of topical application of human breast milk on atopic eczema healing among infants: A randomized clinical trial. J Phil Dermatol Soc 25: 63-66.
- Lee JY, Her Y, Kim CW, Kim SS (2015) Topical corticosteroid phobia among parents of children with atopic eczema in Korea. Ann Dermatol 27: 499-506.
- Bieber T (2008) Atopic dermatitis. N Engl J Med 358: 1483-1494.
- Buescher ES (2001) Anti-inflammatory characteristics of human milk: How, where, why. Adv Exp Med Biol 501: 207-222.
- Murphey DK, Buescher ES (1993) Human colostrum has anti-inflammatory activity in a rat subcutaneous air pouch model of inflammation. Pediatr Res 34: 208-212.
- Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, et al. (2003) The objective severity assessment of atopic dermatitis score: An objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 139: 1417-1422.
- Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, et al. (2003) The objective severity assessment of atopic dermatitis score: An objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 139: 1417-1422.
- Lewis-Jones MS, Finlay AY, Dykes PJ (2001) The infants’ dermatitis quality of life index. Br J Dermatol 144: 104-110.