Journal of Bioelectronics and Nanotechnology
Download PDF
Review Article
*Address for Correspondence: J. H. Markna, Department of Nanotechnology, VVP Engineering College, Rajkot-360005, Gujarat, India, Tel: +91 9974817619; E-mail: jaysukh28@gmail.com
Citation: Pinank K, Kaushik B, Shyam V, Amrutia M, Faldu NU. Revolutionary Therapies and Manipulation of Nanoparticles to Cure Cancer. J Bioelectron Nanotechnol 2016;1(1): 5.
Copyright © 2016 Pinank K et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reviewed & Approved by: Dr. Alexander Marcus Seifalian, Centre for Nanotechnology & Regenerative Medicine, University College London, UK.
Journal of Bioelectronics and Nanotechnology | Volume: 1, Issue: 1
Submission: 06 February, 2016 | Accepted: 03 March, 2016 | Published:07 March, 2016
(iii) Carbon-based nanomaterialsa) Graphene: A particular form of carbon such as graphite is used to prevent cancer. The disadvantage of chemotherapy or radiation is that it can’t restrict damage of healthy cell. The reproducing cells, tumours, and drug resistance are occurring due to cancer stem cells (CSCs) after every treatment [10].
b) Carbon nanotube: Carbon nanotube (CNT) is a one kind of carbon form which is created with the graphene sheet. CNTs have applications in almost all the engineering branches that make new generation materials and devices such as nano dots, nanofiber, and nano needle, etc. Single-walled carbon nanotubes (SWNT) are chemically functionalized with the use of biocompatibility by tumour targeting process, which accumulates in mice and exhibits its cancer therapy process and also decreases excretion and toxicity of tumour cell. A multi-walled carbon nanotube (MWNT) is array, based embedded in ultra-sensitive DNA detector. Thus, carbon nanotube based drug delivery system is used for high treatment efficiency with the minimal side effect. The treatment is schematically shown in Figure 2 [18].
c) CNTs in drug delivery: The main use of the CNTs is as a drug delivery agent. Chemotherapeutic drugs have some limitations because of its noxious side effects. Thus, there grows a need to develop a drug which targets the cell with a wide therapeutic effect. CNT have shown the promising result in the delivery of the drug at targeted area. There have been many experiments performed in vitro and in vivo using functionalized CNTs loaded with chemotherapeutic drugs. Hence, the CNTs are used as the drug delivery is an intravenous injection. One of the problems with injecting drugs is the risk of blocking of blood vessels because of the large size of the drug particles which are likely to damage the tissues. This limitation can be overcome by using the CNTs as carriers for delivering drugs into the body via intravenous injection. Drugs which are to be delivered can either be attached to the surface of the CNTs. This attachment of drug is done via functional groups or drug can be loaded inside the CNTs. Binding of the drug to the outer surface of the CNTs can be done through covalent or non-covalent bonding such as hydrophobic, π-π interaction, electrostatic interactions or Van Der Walls forces [19].
In 1956, Gilchrist who had demonstrated a paper, ‘Selective inductive heating of lymph nodes’, in which he suggested that the particles used in the process whose size was preferable between 20 to 100 nm diameter and also added that we can get 14 °C temperature rise in the time span of only 3 min. In this experiment, various types of nanoparticles can be used but Gilchrist recommended to use the Maghemite particles (Fe2O3). To avoid the reproduction of cancer, hyperthermia generally cooperates with second treatment usability that is supposed to be chemotherapy or irradiation [22,23].
Revolutionary Therapies and Manipulation of Nanoparticles to Cure Cancer
Kacha Pinank1, Babiya Kaushik1, Vasvani Shyam1, Margil Amrutia1, Nehal U. Faldu1, Jay Garach1, Monapara Tushar1, Ghata Bhayani1, Davit B. Dhruv, T. Shiyani1, K. N. Rathod1, Chirag Savaliya1, Ashvini D. Joshi2, Dhiren Pandya3, N. A. Shah3 and J. H. Markna1*
- 1Department of Nanotechnology, VVP Engineering College, Rajkot-360005, Gujarat, India
- 2Department of Electronics and Communication, Government Engineering College, Rajkot-360005, Gujarat, India
- 3Department of Physics, Saurashtra University, Rajkot-360005, Gujarat, India
*Address for Correspondence: J. H. Markna, Department of Nanotechnology, VVP Engineering College, Rajkot-360005, Gujarat, India, Tel: +91 9974817619; E-mail: jaysukh28@gmail.com
Citation: Pinank K, Kaushik B, Shyam V, Amrutia M, Faldu NU. Revolutionary Therapies and Manipulation of Nanoparticles to Cure Cancer. J Bioelectron Nanotechnol 2016;1(1): 5.
Copyright © 2016 Pinank K et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reviewed & Approved by: Dr. Alexander Marcus Seifalian, Centre for Nanotechnology & Regenerative Medicine, University College London, UK.
Journal of Bioelectronics and Nanotechnology | Volume: 1, Issue: 1
Submission: 06 February, 2016 | Accepted: 03 March, 2016 | Published:07 March, 2016
Abstract
Cancer is a malignant neoplastic disease that proliferates throughout the whole body rapidly causing the body to deteriorate and eventually kills the human because of its fatality. Remarkably large number of types of cancers have been discovered by to-day which are named according to their causes and the site of tumour. Cancer spreads quickly across the body. The 1.5 million lives lost per year represent 25% of the estimated 6 million premature cancer deaths that will occur by 2025, and the 6 million figure is itself based on population projections of current numbers and aging. Various therapies have been invented in order to reduce the proliferation pace. Use of nanoparticles such as Au, Ag, Zn nanoparticles in the treatment of cancer into various renowned therapies have been elucidated in the review article. Along with these, the review article has detailed description about two specific therapies namely, Magnetic Hyperthermia and Magnetic Chemotherapy.Keywords
Nanotechnology; Nanoparticles; Nanomedicines; Cancer; Tumour; Magnetic Hyperthermia; Magnetic chemotherapyIntroduction
The following review article has been complied in order to delineate the fatal disease ‘cancer’ and also enlighten the readers about the effect of nanoparticles in treatment of this disease. Origin of cancer has always fascinated mankind. When there is an uncontrollable cell growth in the human body, tumours grow rapidly which is identified as cancer because these cells deteriorate that specific site in the body. The maneuvering of tumours inside the body is called metastasization [1-4]. This malignant effect has been characterized by various high level medical researches and clinical trials. Cancer cells spread enormously in the body throughout all the blood vessels. Traditional treatments are surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy, biological therapy. Among these treatment techniques, surgery can include the use of microelectromechanical devices or nanoelectromechanical devices [2]. One of the few therapies is chemotherapy which is probably not recommendable because it has unbearable side effects on the human body though it has been the only cure since the near past [3-5]. Discussing about the targeted therapy, we can focus on its advantage that it has a notable control over hair loss caused by chemotherapy; this technique can be applied to cancers such as breast cancer, multiple myeloma, lymphoma, prostate cancer, and melanoma [6,7]. Other therapies viz. radiation therapy, hormonal therapy, photodynamic therapy have been invented over time with betterments and reduced limitations [8,9].Nanoparticles for Cancer Treatment
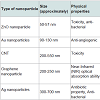
The conventional cancer treatment has some limitations to cure cancer which can be resolved by using nanoparticles of ZnO, silver, gold, graphene, CNT, etc. for cancer treatments as discussed below.(i) ZnO nanoparticles
ZnO nanoparticles are very effective and can be used to destroy the three types of cancer cells: (i) human hepatocellular carcinoma HepG2, (ii) human lung adenocarcinoma A459, (iii) human bronchial epithelial BEAS-2B. ZnO nanoparticles show various effects on mammalian cell feasibility through termination of all three types of cancer cells while characterizing on normal rate cells such as astrocytes and hepatocytes. The toxicity appliance of ZnO nanoparticles was examined using human liver cancer HepG2 cells. The poisonous effects of ZnO nanoparticles are due to the immovability of compounds [10].
The nontoxicity of ZnO nanoparticles is the main aspect to use it as an antibacterial material. The toxic effect of ZnO nanoparticles on T-cells and neuroblastoma cells is at a concentration of 5 mm and 1.2 mm, respectively. Nontoxicity, which is indicated by the normal osteoblast function, is improved by ZnO nanoparticles. The anticancer property of ZnO nanoparticles in a medical field is an underdeveloped research area [10].
Synthesis of ZnO nanoparticles: The nanoparticles of ZnO can be synthesis by many processes such as Ball milling or Sol-gel method. The ZnO nanoparticles were synthesized from Zinc Acetate Dihydrate and Oxalic Acid were mixed. They were later ground in mortar and pestle for 60 min at room temperature. The molar concentration ratio of the reactants Zinc Acetate Dihydrate to Oxalic Acid taken was 1:1.5. The chemical reaction between the compounds activated by grinding the mixture, Zinc Oxalate is obtained as an intermediate product. The intermediate product was then annealed at 4500 °C in a furnace for removing CO, CO2 and moisture from the compound. The thermal decomposition of Zinc Oxalate at 4500 °C resulted in obtaining Zinc Oxide nanoparticles.
ZnO nanoparticles were with a size between 15 to 20 nm and spherical; they were successfully synthesized by ball milling method. The study shows the potential anticancer activity of ZnO nanoparticles on the cancer cell. The synthesis process for large scale production of ZnO was economically feasible, biocompatible and cost-effective. The results show the use of the oxide-based nanoparticles as chemotherapeutic agents which would have a wide range of applications in the treatment of cancerous cells. The variation in toxicity of ZnO nanoparticles can be obtained by varying the size of bulk ZnO nanoparticles [11].
(ii) Gold nanoparticles
Gold nanoparticles are biocompatible material for the medical purpose. Nanoparticles mostly in size of 1-100 nm, can be injected into the vein for targeted drug treatment. According to types of cells, selective gold nanoparticles will be attached to desired antibodies. This type of treatment uses less medication with no side effects. This form of cancer cells help to absorb substantially higher concentration of nanoparticles than surrounding tissues [12,13].
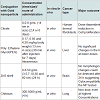
Table 2: Summary of gold nanoparticles with different conjugation used in cancer treatmentAbove tabular form of data gives the clear idea about the way specific nanoparticles react with antibodies or the certain cancerous cells. In vitro processes take place outside the living body while in vivo processes are carried out inside the living being. Concentrations of certain nanoparticles required to cure specific cancer are also mentioned in the table.
When CSCs are converted into a tumour-sphere or in a new tumour cell, they have stress resistance, rapidly dividable and immortal. The oxidized carbon such as graphene oxide (GO) is soluble in many solvents. Graphene oxide is experienced in many types of cancers such as breast cancer, pancreatic cancer, lung cancer, brain cancer, ovarian cancer, prostate cancer and skin cancer [14]. Graphene oxide forces conversion of non-cancer stem cells from active cancer stem cells and also prevents future tumours from the roots.
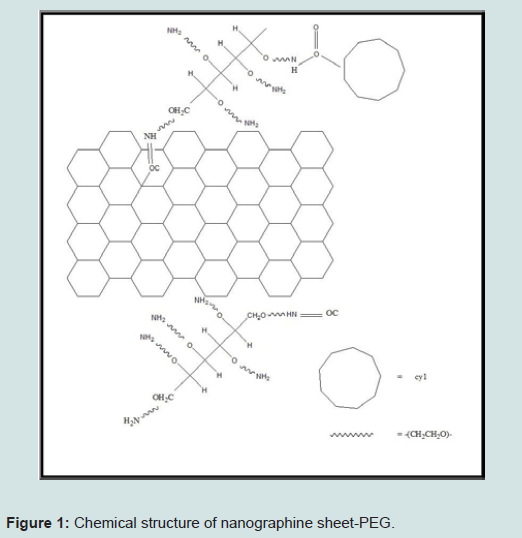
The nanographene sheet coated with polyethylene glycol (PEG) can be fabricated in a laboratory and can be used in medical application, especially in the cancer treatment. Graphene has lots of applications in many areas such as electronics, biomedical, nanotechnology, etc. The utilization of strong near-infrared (NIR) optical absorption ability of Nano Graphene Sheet (NGS) for the photothermal therapy of cancer can be used for high tumour destruction [15]. Furthermore, we do not get the mark of poisonous effect for NGS-PEG injected mice according to the observations on its body. This additional advantage of NGS with a biocompatible coating is due to the 2-D nanometers which have an excellent potential in cancer therapy [16].
Fabrication of nanographene sheet-PEG starts with a basic component of this process that is graphite oxide. Hsin-Ying Wu et al. had synthesized PEG using hydrochloric acid and the N-hydroxy ULF succinimide sodium salt, 2-(N-Morpholino) Ethane Sulfonic Acid Hydrate (MES) buffer solution was taken as a base. Then 0.2 ml aliquots of this prepared solution were mixed with GO-COOH and this precursor was kept at the certain temperature for an hour in the dark, resulting in a formation of amide bonds between activated carboxyl groups [17]. Then activated GO-COOH were divided and washed with MES buffer. This process is repeated by dissolving in MES buffer and then mixed with PEG-NH2 and kept at the specific temperature for 120 min. PEG-graphite oxide was divided from solution and centrifuged to remove both MES buffer [17].
Peroxidase-conjugated antimouse IgM, IgG1, IgG2a, or IgE was added and incubated for 60 min. The plates were washed again and the bound peroxidase conjugate was detected by the addition of the tetramethylbenzidine substrate solution. Microplate reader shows optical density value of 450 nm using a microplate reader. We can get structures as shown in Figure 1 after the compilation of synthesis process [17].
b) Carbon nanotube: Carbon nanotube (CNT) is a one kind of carbon form which is created with the graphene sheet. CNTs have applications in almost all the engineering branches that make new generation materials and devices such as nano dots, nanofiber, and nano needle, etc. Single-walled carbon nanotubes (SWNT) are chemically functionalized with the use of biocompatibility by tumour targeting process, which accumulates in mice and exhibits its cancer therapy process and also decreases excretion and toxicity of tumour cell. A multi-walled carbon nanotube (MWNT) is array, based embedded in ultra-sensitive DNA detector. Thus, carbon nanotube based drug delivery system is used for high treatment efficiency with the minimal side effect. The treatment is schematically shown in Figure 2 [18].
c) CNTs in drug delivery: The main use of the CNTs is as a drug delivery agent. Chemotherapeutic drugs have some limitations because of its noxious side effects. Thus, there grows a need to develop a drug which targets the cell with a wide therapeutic effect. CNT have shown the promising result in the delivery of the drug at targeted area. There have been many experiments performed in vitro and in vivo using functionalized CNTs loaded with chemotherapeutic drugs. Hence, the CNTs are used as the drug delivery is an intravenous injection. One of the problems with injecting drugs is the risk of blocking of blood vessels because of the large size of the drug particles which are likely to damage the tissues. This limitation can be overcome by using the CNTs as carriers for delivering drugs into the body via intravenous injection. Drugs which are to be delivered can either be attached to the surface of the CNTs. This attachment of drug is done via functional groups or drug can be loaded inside the CNTs. Binding of the drug to the outer surface of the CNTs can be done through covalent or non-covalent bonding such as hydrophobic, π-π interaction, electrostatic interactions or Van Der Walls forces [19].
(iv) Silver nanoparticles
Silver (Ag) nanoparticles are emerging as promising agents for cancer treatment. Silver nanoparticles are very efficient than the other nanomaterials because the toxicity of silver nanomaterial is used for killing cancer cells. It is very noxious for Dalton’s Lymphoma Ascites (DLA) cell. S. Grandiflora (L.) is the natural source which is used to synthesize Ag nanoparticles. The plant has been widely used in conventional medicines as well as in advanced medicines for the treatment of diseases viz. tumour and liver disorders [19]. S. Grandiflora (L.) leaf extract was made by mixing 20 g of leaf powder in 200 ml distilled water. Then the solution was kept in boiling water bath for 10 min. The extract was filtered through Whattman filter paper and then it was stored at 4 °C. Ag nanoparticles were prepared by mixing 10 ml plant extract in 90 ml of 1 mM AgNO3 and kept at specific temperature. After addition of the leaf extract, reduction of silver ions was obtained after 24 h of incubation at 37 °C. Silver nanoparticles had been observed due to the formation of yellowishbrown colour. Synthesis of Ag nanoparticles was confirmed by UVvisible spectroscopy in the range between 300-700 nm.
Ag nanoparticles were injected into a nearby region of the cancerous cell through injection. Ag nanoparticles react with the cancer cell and deactivate them. The cancer risks get reduced effectively after injecting silver nanoparticles for 4 to 5 times. The process is slow but it can treat the cancerous cells from the body [19].
Magnetic Nanoparticles for Cancer
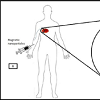
Under the influence of alternating current (AC) magnetic field, the nanoparticles produce heat by Neel and Brownian relaxation. Basically, the hyperthermia term is clinically understood as getting of temperature increment in the human body as compared to normal also in general, it is used in cancer treatment for annihilation of tumour [20]. When the magnetic nanoparticle is caught at desired region of the human body, specifically at tumours it can be said that hyperthermia is spontaneously acquired. The main advantage is that it particularly increases the temperature of Nanoparticle-containing tissues. The following Figure 3 gives an understanding about the magnetic hyperthermia [21,22].
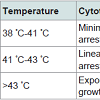
Table 3: Summary of Hyperthermia effect with different temperature stages.Above table has been precisely observed and concluded in order to get a clear idea about the various stages occurring in the Hyperthermia therapy. As defined earlier, Magnetic hyperthermia is the therapy that deals with varying temperature near the tumour because of alternating magnetic field. When the magnetic field is alternated continuously, the magnetic nanoparticles that have agglomerated near the tumour vibrate i.e. flip orientation; this leads to a heating effect at the certain site which gives an increment in the amount of heat that eventually destroys the tumour without leaving behind any defective cells.
The use of magnetic hyperthermia is recently derived from a cancer treatment but it needs to use improved magnetic materials to enhance the chances of succeeding a therapy. Also, the patient’s blood circulation flow has to be taken into consideration that includes controlled temperature rise. Some materials like superparamagnetic materials can be used because of their properties. They have enormous potential that they would have their domains aligned in the same direction in the presence of external magnetic field while in theabsence of magnetic field, their domains act as individual particles, which remain inert. To implement those nanoparticles in the cancertreatment, the clinical testing was reported with proof by Jordan’s magnetic field therapy system [22].
The magnetic hyperthermia is very much dependable on the cancer type because the relative flow of blood at a particular region of the human body can vary. Various cancerous cells can have a different kind of parameters and also have a particular range of magnetic field sensitivity for its destruction and due to these reasons, the cure of cancer is very expensive and very difficult [22,24].
b) Magnetic chemotherapy
The magnetic drug can be used in the treatment if the tumour is connected to the arterial system from where the blood is supplied to the tumour. Thus, anticancer drug targeted in the tumour directly with fewer side effects, and the magnetic drug delivery system is less toxic than other treatments [25].
When the magnetic particle reaches its targeted area then it starts to release the drug from the magnetic particle near region of the cancerous cell. This process of releasing drug from the magnetic particle occurs due to the change in pH, concentration difference between the particle and blood/tissue. Wider and Senyei were the first to give the concept of the drug-carrying magnetic nanoparticle. The scientist had encapsulated chemotherapeutic drug doxorubicin in the albumin coated magnetite particle whose size is around 1 to 2 micrometer. Wider and Senyei had targeted the distinct area in the rat tail and then delivered the drug using magnetic particle, the result whose 200 times better than the traditional method of the drug delivering [25].
The magnetic albumin microsphere which is used as the drug carrier was never tested in the humans because the magnetophoretic mobility was too low for the internal organ of the body. To overcome this limitation the iron-carbon particle is developed by the FeRx. They had prepared the irregularly shaped carbon-coated iron particle in 1 to 5 micrometer and which has a high magnetic susceptibility. This carbon-coated iron particle is loaded with doxorubicin and result was highly promising in the treatment of liver cancer [25].
From the result of the doxorubicin, many other drugs are tested with other matrix materials. For example, the poly alkyl cyanoacrylate nanoparticles whose size around 200 nm are filled with dactinomycin, ferrocarbon nanoparticle whose size is around 100 nm diameter is loaded with the carminomycine, solid lipid nanoparticle whose size is around 460 nm to 550 nm in diameter is filled with the methotrexate. These all drug had shown the positive result after manipulating the magnetic drug particle to its targeted area and release the drug in proper concentration on the tumour cells [25].
Then the scientist had tried the intravenous injection of magnetic particle directly to the targeted region. The result was more promising then the manipulation by the magnetic field. The result shows that the 50% of the magnetic drug remained near the target region in the manipulating process by a magnetic field. The magnetic drug was not able to reach at targeted region due to lower magnetic susceptibility and size. Thus, intravenous injection of the magnetic particle into the blood is more promising then manipulating by the magnetic field.
c) Fe2O3 nanoparticle as drug carrier
In the magnetic nanoparticle generally iron is used due to its magnetic property. Therefore, the two types of iron compounds are used such as magnetite (Fe3O4) and maghemite (α-Fe2O3). These compounds are superparamagnetic in nature so that the iron compound can easily manipulate through a magnetic field and they also show the biocompatibility property due to which they are used as a drug carrier.
Massart aqueous precipitation method is used to prepare waterdispersible iron oxide magnetic nanoparticle. During this process, the size of the magnetic nanoparticle can be changed from 4 to 30 nm range. The shape of the particle which is obtained in the process is generally ellipsoidal [25].
There are many methods used for the synthesis of the iron oxidebased nanoparticle. Further, many modifications can be done in the structure of magnetite and maghemite by adding nickel and cobalt atom during the synthesis process [25-29].
Conclusion and Potential Directions
The above reviews on cancer treatment have been elaborated in order to reduce the deadly impact of the fatal disease named cancer on the world. With the help of the therapies discussed in the article, one can accurately demolish this disease painlessly from the human body restoring the healthy body. Highly efficient nanoparticles have been of great help to scientists and doctors as drug carriers to overcome cancer by targeted drug delivery.References
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13: 607-615.
- Paula G (2011) Surgical robotics and computer-integrated manufacturing. J Robotics 27: 261-266.
- Wang CY, Cusack JC Jr, Liu R, Baldwin AS Jr (1999) Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 5: 412-417.
- Madhav NV, Shakya AK, Shakya P, Singh K (2009) Orotransmucosal drug delivery systems: a review. J Control Release 140: 2-11.
- Michael M, Tannock IF (1998) Measuring health-related quality of life in clinical trials that evaluate the role of chemotherapy in cancer treatment. CMAJ 158: 1727-1734.
- Misra R, Acharya S, Sahoo SK (2010) Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discovery Today 15: 842-850.
- Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumor immunity. Nat Rev Cancer 6: 535-545.
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, et al. (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280: 969-974.
- DeVita VT, Hellman S, Rosenberg SA (2005) Cancer: principles & practice of oncology. Philadelphia: Lippincott.
- Rasmussen JW, Martinez E, Louka P, Wingett DG (2010) Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 7: 1063-1077.
- Selvakumari D, Deepa R, Mahalakshmi V, Subhashini P, Lakshminarayan N (2015) Anti cancer activity of ZnO nanoparticles on MCF7 (breast cancer cell) and A549 (lung cancer cell) 10: 5418-5421.
- 12. Huang X, Jain PK, El-Sayed IH, El-Sayed MA (2008) Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23: 217-228.
- Yildirimer L, Thanh NT, Loizidou M, Seifalian AM (2011) Toxicological considerations of clinically applicable nanoparticles 6: 2963-2979.
- Vinogradov S, Wei X (2012) Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond) 7: 597-615.
- Brandt O, Mildner M, Egger AE, Groessl M, Rix U, et al. (2012) Nanoscalic silver possesses broad-spectrum antimicrobial activities and exhibits fewer toxicological side effects than silver sulfadiazine. Nanomedicine 8: 478-488.
- Yang K, Zhang S, Zhang G, Sun X, Lee ST, et al. (2010) Graphene in mice: ultrahigh in vivo tumour uptake and efficient photothermal therapy. Nano Lett 10: 3318-3323.
- Wu HY, Lin KJ, Wang PY, Lin CW, Yang HW, et al. (2014) Polyethylene glycol-coated graphene oxide attenuates antigen-specific IgE production and enhanced antigen-induced T-cell reactivity in ovalbumin-sensitized BALB/c mice. Int J Nanomedicine 9: 4257-4266.
- Madani SY, Naderi N, Dissanayake O, Tan A, Seifalian AM (2011) A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomedicine 6: 2963-2979.
- Jeyaraj M, Sathishkumar G, Sivanandhan G, MubarakAli D, Rajesh M, et al. (2013) Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surfaces B Biointerfaces 106: 86-92.
- Ruddy K, Mayer E, Patridge A (2009) Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59: 56-66.
- Hafeli UO (2006) The history of magnetism in medicine. In: Andra W and Nowak H, (Eds). Magnetism in medicine: A Handbook, (2nd edn), Wiley-VCH: Berlin, pp. 1-25.
- Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, et al. (2006) Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J 35: 446-450.
- Shinkai M (2002) Functional magnetic particles for medical application. J Biosci Bioeng 94: 606-613.
- Sheng R, Flores GA, Liu J (1999) In vitro investigation of the novel cancer therapeutic method using embolizing properties of magnetorheological fluids. J Magn Magn Mater 194: 167-175.
- Torchilin VP (2006) Nanoparticulates as drug carriers.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277-300.
- Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8: 147-166.
- Hong SC, Lee JH, Lee J, Kim HY, Park JY, et al. (2011) Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int J Nanomedicine 6: 3219-3231.
- Madani SY, Tan A, Dwek M, Seifalian AM (2012) Functionalization of single-walled carbon nanotubes and their binding to cancer cells. Int J Nanomedicine 7: 905-914.