Journal of Andrology & Gynaecology
Download PDF
Research Article
*Address for Correspondence: Pratiksha Gupta, MD, Department of Gynecology and Obstetrics, Post Graduate Institute of Medical Sciences and research, ESIC Model Hospital, Basaidarapur, Ring Road, New Delhi-110015, India, Tel: +919871128703; E-mail: drpratiksha@gmail.com
Citation: Gupta P, Sunita J and Shekhar C. Misoprostol versus Oxytocin in Prevention of Postpartum Hemorrhage. J Androl Gynaecol. 2016;4(1): 4.
Copyright © 2016 Gupta P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN : 2332-3442 | Volume: 4, Issue: 1
Submission: 08 February, 2016| Accepted: 25 March, 2016 | Published: 29 March, 2016
Misoprostol versus Oxytocin in Prevention of Postpartum Hemorrhage
Pratiksha Gupta*, Sunita Jindal and Chandna Shekhar
- Department of Gynecology and Obstetrics, Post Graduate Institute of Medical Sciences and research, India
*Address for Correspondence: Pratiksha Gupta, MD, Department of Gynecology and Obstetrics, Post Graduate Institute of Medical Sciences and research, ESIC Model Hospital, Basaidarapur, Ring Road, New Delhi-110015, India, Tel: +919871128703; E-mail: drpratiksha@gmail.com
Citation: Gupta P, Sunita J and Shekhar C. Misoprostol versus Oxytocin in Prevention of Postpartum Hemorrhage. J Androl Gynaecol. 2016;4(1): 4.
Copyright © 2016 Gupta P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN : 2332-3442 | Volume: 4, Issue: 1
Submission: 08 February, 2016| Accepted: 25 March, 2016 | Published: 29 March, 2016
Abstract
Study background: The present study was done to compare oxytocin and misoprostol in active management of third stage of labor.Materials and methods: Total of 200 pregnant women were enrolled in the study were divided into two randomized groups. Group ‘A’ included 100 primigravidas with singleton pregnancy and normal vaginal delivery, who received 600μg misoprostol sublingually with delivery of baby. Group ‘B’ included equal number of primigravidas with singleton pregnancy, who underwent normal vaginal delivery and received 10 IU intramuscular oxytocin after delivery of anterior shoulder of baby.
Results: The fall in hemoglobin was ≥ 1 g/dl in 6% of oxytocin group and 8% of misoprostol group and mean blood loss was comparable in the two groups (p = 0.455). None of the cases had mean amount of blood loss ≥ 1000 ml. Incidence of postpartum hemorrhage within an hour of delivery in misoprostol and oxytocin group was 8% and 6% respectively which was comparable (p = 0.435). Occurrence of nausea, vomiting, diarrhea, were also not significantly different between groups (p = 0.102, 0.071, 0.700 respectively) while fever (≥ 38 °C) and shivering was significantly present more in misoprostol group (p = 0.001). Vertigo was present in 2% of misoprostol and 3% of oxytocin group and the difference was not significant (p = 0.651).
Conclusions: Misoprostol and oxytocin both were equally effective in prevention of postpartum hemorrhage Though shivering and pyrexia are its specific side effect but they were transient; thus Misoprostol appears to be a safe, inexpensive, thermostable, long shelf life without special storage and effective uterotonic for use in rural and remote areas where parentral oxytocin may be unavailable. It can be given to birth attendants for routine use in third stage of labor especially in rural settings.
Keywords
Misoprostol; Oxytocin; Postpartum hemorrhage; LaborIntroduction
Postpartum hemorrhage (PPH) continues to be the prime cause of maternal deaths predominantly in countries with limited resources [1]. PPH is mainly related to uterine atonicity therefore WHO has recommended active management of the third stage of labor [2]. Execution of WHO guidelines in rural areas and in developing countries including India poses immense problems, as storage and parenteral administration of an oxytocic by a trained health worker is not feasible at many times in rural set up. Even lack of refrigeration for storing parenteral uterotonic drugs and non-availability of sterile syringes and needles, also poses a major problem. Therefore, there is a need for a safe, effective, affordable, thermo-stable, and non-parenteral uterotonic drug. Misoprostol is stable at room temperature, affordable and easy to administer. The present study was undertaken to assess the average blood loss in third stage of labor and find out the incidence of PPH in misoprostol group and oxytocin group. Side effects produced by these drugs were also compared.Materials and Methods
It was a prospective randomized study, was conducted in the labor room, Department of Obstetrics and Gynecology, PGIMSR, ESIC during one year period from November 2009 to November 2010. 200 pregnant women were enrolled and were divided into two groups.Group ‘A’ included 100 pregnant women who were primigravidas with singleton pregnancy, and underwent normal vaginal delivery. They received 600 μg misoprostol sublingually as soon as baby was delivered.
Group ‘B’ included 100 pregnant women who were primigravidas with singleton pregnancy, and underwent normal vaginal delivery. They received 10 IU intramuscular oxytocin with the delivery of anterior shoulder of baby. Inclusion criteria was all primigravidas with singleton pregnancy who had normal vaginal delivery and exclusion criteria was multiparous women, multiple gestations, medical disorder, uterine malformation, induced labor and use of other uterotonic agents. After detailed patient’s history and examination women fulfilling the inclusion criteria were enrolled in the study group with written informed consent. Normal hematological investigations were done including hemoglobin and hematocrit at the time of admission and 48 hours after delivery. When vaginal delivery was eminent, episiotomy was given before delivery of baby and the doctor picked an envelope for assigning the particular group and sequentially numbered sealed envelopes were kept indicating the method of treatment of third stage of labor to which the women was allocated. Each envelope had a card with instruction for either group. Those marked with “M” indicated randomization to receive 600 μg misoprostol sublingually immediately after birth of the baby and after division of umbilical cord (Group A).
Cards marked with “O’ indicated randomization to the standard policy of giving intramuscular 10.
IU oxytocin, which was administered intramuscularly at the delivery of anterior shoulder (Group B). Placenta was delivered by modified Brandt Andrew’s technique. Placenta was carefully examined for completeness. Within a minute of delivery linen soiled with amniotic fluid was removed, a new disposable absorbent linen sheet was placed under the women and a sterile pan applied against the women buttocks to collect all blood. After stitching episiotomy the pads, linen, and pan were removed and blood loss was measured by subtracting the dry weight to give the approximate volume of blood in milliliters. In case of traumatic PPH the tear was sutured and the cases were excluded from the study. In non-traumatic atonic PPH bimanual massage of the uterus was done and uterotonic were given and managed accordingly. Variables concerning labor and delivery were recorded such as duration of all stages of labor, mode of delivery, episiotomy, tears, atonic PPH, need for additional oxytocics regimen and manual removal of placenta were recorded. Side effects such as nausea, vomiting, diarrhea, fever, shivering, vertigo, and headache were recorded one hour after delivery. Outcome measures were calculated as primary outcome - Maternal hemoglobin and hematocrit measured on admission to labor room and repeated 48 hours after delivery, and amount of blood loss during third stage of labor. Secondary outcome was measured in terms of duration of third stage of labor, manual removal of placenta, additional oxytocics given and side effects one hour after delivery that is nausea, vomiting, diarrhea, fever, shivering, vertigo, and headache. Data were analyzed and expressed as mean ± SD. Chi-square test was done to compare the categorical variables among the groups and Mann Whitney test for presenting the continuous variable. P value of ≤ 0.05 was taken as significant.
Results
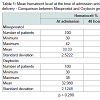
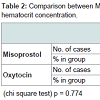
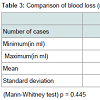
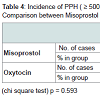
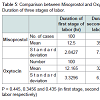
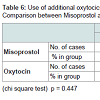
Demographic profile was comparable between the groups. Mean age for misoprostol and oxytocin group was 22.86 years and 22.02 years respectively (p = 0.445) showing comparable age distribution between the two groups. The birth weight of the baby among PPH and non PPH cases in both groups were also comparable. Mean hemoglobin level at the time of admission among the misoprostol group and oxytocin group were 11.261 g/dl and 11.210 g/dl and (P = 0.650) with no significant difference between the two groups. Mean hemoglobin value after 48 hour of delivery was comparable between misoprostol and oxytocin groups (10.939 g/dl and 11.011 g/dl) and no significant difference noted between the groups (p = 0.546). The fall in hemoglobin value of ≥ 1 g/dl was present in 6% of oxytocin group and 8% of misoprostol group and the difference was not significant. There was also no significant difference between oxytocin and misoprostol groups in mean hematocrit level at the time of admission (32.980% and 33.33% respectively, p = 0.290) and at 48 hours after delivery (32.569% and 32.587% respectively, p = 0.958). The fall in hematocrit concentration of ≥ 3% was present in 6% of oxytocin group and 7% of misoprostol group. There was no significant difference between the two groups in the amount of blood loss (125.10 ± 156.276 ml v/s 124.14 ± 151.846 ml). The mean blood loss between the two groups were comparable (p = 0.455) (Tables 1-3). Incidence of PPH in misoprostol group was 8% of case while it was 6% in oxytocin group with no significant difference. In either group none of the cases had mean amount of blood loss ≥ 1000 ml (Table 4). Mean duration of first stage of labor (12.5 v/s 12.165 hours, p = 0.445) second stage of labor (35.270 v/s 32.400 min, p = 0.356) and third stage of labor (4.325 v/s 4.025 min, p = 0.435) were comparable between the misoprostol and oxytocin groups (Table 5). Manual removal of placenta was not required in any of the women in either group. Additional oxytocics were required in 10% of patients in misoprostol group and 7% of patients in oxytocin group with no significant difference (p = 0.447) (Table 6). The additional oxytocics required were 10 U intravenous oxytocin (dissolved in 500 ml ringer lactate solution) and injection methylergometrine (0.2 mg intravenously or intramuscularly). Nausea, vomiting, diarrhea, were not different significantly between the groups (p = 0.102, 0.071, 0.700 respectively). Fever (≥ 38 °C) was present more in misoprostol group than in oxytocin group and was highly significant (p = 0.001).Vertigo was present in 2% of misoprostol and 3% of oxytocin group and the difference was not significant (p value 0.651).
Discussion
In present study the women belonged to lower age group (22.86 years) because women marry and reproduce early in India, in contrast to other studies [3-5]. In this study the mean hemoglobin level at the time of admission was 11.6 gm% and at 48 hours after delivery it was 10.93 gm%. The hemoglobin difference of more than 1 gm% was present in 8% of misoprostol group. In one study mean pre-delivery hemoglobin was 12.7 gm% and post-delivery hemoglobin was 10.9 gm%, mean fall in hemoglobin was 1.6 gm% and incidence of postpartum hemorrhage (≥ 500 ml) and postpartum blood loss in the misoprostol group were similar to those in the oxytocin group (6% versus 5.7%, p = 0.85; 153 ml versus 146 ml, P = 0.36) [3]. Shivering and pyrexia were encountered more often in the misoprostol than in the oxytocin group (shivering: 19% versus 0.8%, P < 0.001, relative risk [RR] 0.86, 95% confidence interval [CI] 0.82-0.90; pyrexia: 2.3% versus 0%, P = 0.03, RR 0.97, 95% CI 0.95-0.99) [6].In another study patients who received 600 μg of misoprostol had the lowest blood loss (96.05 ± 21.1 ml), followed by 400 μg of misoprostol (126.24 ± 49.3 ml), oxytocin (154.7 ± 45.7 ml), and methylergometrine (223.4 ± 73.7 ml) (P < 0.01). The 48-hour postpartum hemoglobin level was similar among the groups (P > 0.05). Shortest mean duration of the third stage of labor was with 600 μg of misoprostol. Pyrexia was observed in the misoprostol groups, and raised blood pressure in the methylergometrine group (P < 0.001). Oxytocin induced least adverse effects. They concluded that administration of 600 μg of sublingual misoprostol was more effective than 400 μg of misoprostol, intravenous oxytocin, and intravenous methylergometrine [7].
Present study is comparable with one study, in which PPH was present in 5.8% of cases [8]. A recent multicenter trial found that 600 μg of oral misoprostol was superior to conventional oxytocics such as methylergometrine [4]. Oral misoprostol has been found to have comparable results to standard parenteral oxytocics in reducing PPH [4,9,10]. However, conflicting results showing that misoprostol is less effective than traditional uterotonics have also been published [11]. A recent Cochrane meta-analysis concluded that misoprostol is better when compared the duration of the third stage of labor, blood loss, and adverse effects of other agents. Although misoprostol has been used in a few studies, either at 400 μg or 600 μg, no studies have compared the two doses [4,12-14].
The sublingual route of administration of misoprostol was chosen in the present study because of better pharmacokinetics compared with oral and was as effective as conventional parenteral oxytocics. Sublingual administration of misoprostol has been used as a prophylactic oxytocic in a few studies. Sublingual misoprostol at a dose of 400 μg has been shown to result in significantly lower blood loss compared with 20 IU of oxytocin infusion during cesarean delivery [15].
In present study mean duration of third stage of labor was 4.325 minutes. None of our patients have duration of third stage of labor to be ≥ 30 minutes, whereas in studies the duration was 7.9 minutes, > 30 minutes, 11-30 minutes, 8 minutes respectively [5,9,12,14]. Manual removal of placenta was not required in our study, similar to other studies [3,5]. In present study use of additional oxytocics was around 10% and is comparable with few studies [3-5,12,16].
Transient pyrexia and shivering were the two most common adverse effects seen in present study. Transient pyrexia was mostly seen with misoprostol, as has been reported previously [8,16-18].
Research suggests that within rural health settings, sublingual misoprostol can effectively be integrated into local practice and can significantly reduce the incidence of PPH and the need for emergency patient transfer and emergency obstetric care (blood transfusion and surgical intervention. In rural areas of India where expectant management of labor is practiced, a PPH rate of approximately 12% can be anticipated [14]. This high prevalence further emphasizes the need for primary preventive efforts through the provision of an easy to use, stable uterotonic agent such as misoprostol.
Conclusion
Sublingual misoprostol is as effective as intramuscular oxytocin in prevention of postpartum hemorrhage but with higher side effects of transient pyrexia and shivering. Misoprostol appears to be a safe, inexpensive and effective uterotonic for use in developing countries with less resources in rural, remote and difficult areas for active management of third stage of labor where intramuscular oxytocin is not practical because of the need for proper storage, protection from light, need for refrigeration and parentral administration by skilled personnel. Misoprostol is effective, thermostable, easily administered by oral route and has long shelf life without special storage. The present study includes small number of cases and so larger studies are required to assess the use of misoprostol in women at risk of postpartum hemorrhage and it can be given to birth attendants for routine use in third stage of labor especially in rural settings. This definitely will reduce maternal morbidity and mortality.References
- Li XF, Fortney JA, Kotelchuck M, Glover LH (1996) The postpartum period: the key to maternal mortality. Int J Gynaecol Obstet 54: 1-10.
- Mathai M, Gülmezoglu AM, Hill S (2007) Saving women’s lives: evidence-based recommendations for the prevention of postpartum haemorrhage. Bull World Health Organ 85: 322-323.
- Baskett TF, Persad VL, Clough HJ, Young DC (2007) Misoprostol versus oxytocin for the reduction of postpartum blood loss. Int J Gynaecol Obstet 97: 2-5.
- El-Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, et al. (2000) The misoprostol third stage of labor study: a randomized controlled comparison between orally administered misoprostol and standard management. BJOG 107: 1104-1110.
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC (2006) Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. Int J Gynaecol Obstet 92: 170-175.
- Chaudhuri P, Biswas J, Mandal A (2012) Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. Int J Gynaecol Obstet 116: 138-142.
- Singh G, Radhakrishnan G, Guleria K (2009) Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. Int J Gynaecol Obstet 107: 130-134.
- Kundodyiwa TW, Majoko F, Rusakaniko S (2001) Misoprostol versus oxytocin in the third stage of labor. Int J Gynaecol Obstet 75: 235-241.
- Cook CM, Spurrett B, Murray H (1999) A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labor. Aust N Z J Obstet Gynaecol 39: 414-419.
- Joy SD, Sanchez-Ramos L, Kaunitz AM (2003) Misoprostol use during the third stage of labor. Int J Gynaecol Obstet 82: 143-152.
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, et al. (2005) Misoprostol in the management of the third stage of labor in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG 112: 1277-1283.
- Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, et al. (2001) A multicentre randomized controlled trial of oral misoprostol and i.m. syntometrine in the management of the third stage of labor. Hum Reprod 16: 31-35.
- Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, et al. (2008) Factors associated with acute postpartum hemorrhage in low-risk women delivering in rural India. Int J Gynaecol Obstet 101: 94-99.
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, et al. (2006) Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 368: 1248-1253.
- Vimala N, Mittal S, Kumar S (2006) Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynaecol Obstet 92: 106-110.
- Gulmezoglu AM, Forna F, Villar J, Hofmeyr GJ (2007) Prostaglandins for prevention of postpartum hemorrhage. Cochrane Database Syst Rev: CD000494.
- Lam H, Tang OS, Lee CP, Ho PC (2004) A pilot-randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstet Gynecol Scand 83: 647-650.
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S (2004) Sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Int J Gynaecol Obstet 87: 1-5.